Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Gout is the most common inflammatory arthritis and is particularly common among veterans. Recent studies, a randomized controlled trial (CARES, White et al. NEJM 2018) and an observational study using administrative claims data (Zhang et al. Circulation 2018) point to a higher all-cause and cardiovascular mortality with febuxostat relative to allopurinol. However, the CARES trial was limited to patients with a history of major cardiovascular disease and 45% subjects were lost to follow up. Therefore, the objective of our study was to evaluate the comparative risk of cardiovascular events in veterans with gout on allopurinol versus febuxostat.
Methods: We identified patients age >=18 with gout who initiated allopurinol during 2009-2017 using encounter and claims data from the Corporate Data Warehouse housed within the Veteran Affairs Informatics and Computing Infrastructure (VINCI). The Index date was the date of first allopurinol fill. Patients were followed in monthly increments for up to 8 years and, for patients who subsequently initiated febuxostat, the date of first febuxostat fill was noted. Exclusion criteria included patients with end stage renal disease or on dialysis; use of pegloticase or rasburicase in the 365 days before the index date. The primary outcome was development of major adverse cardiovascular events (MACE) defined as hospitalization for myocardial infarction (MI), stroke (including TIA), or heart failure (new-onset heart failure or heart failure exacerbation). The secondary outcomes were myocardial infarction, stroke, new and recurrent heart failure, and all-cause mortality. Marginal structural models (MSM) were used to determine the hazard of MACE events with febuxostat relative to allopurinol while accounting for changes in patient characteristics over time that influence the use of febuxostat (serum uric acid and glomerular filtration rate). Censoring events included death, date of last available allopurinol or febuxostat, or end of follow up (whichever comes first).
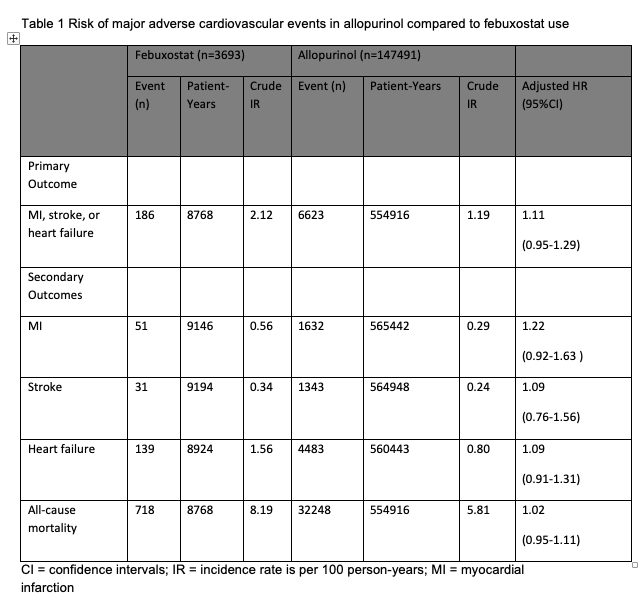
Results: We identified 151,184 new allopurinol users, of which 3,693 (2.4%) subsequently initiated febuxostat over the study period. Allopurinol-only users were predominantly male (98.4%) and white (63.7%), respectively, with similar attributes in those switching to febuxostat. 56% of allopurinol users had uric acid >8-< 12 mg/dl compared to 70.3% of febuxostat group at drug initiation. There were 186 MACE events over 8,768 patient years of febuxostat use (crude incidence rate (IR)=2.12/100 patient-yrs) compared to allopurinol 6,623 events over 554,916 patient years (crude IR=1.19/100 patient-yrs); adjusted hazard ratio (HR) being 1.11 (95% CI 0.95-1.29, p=0.19) after multivariable adjustment. We also observed no statistically significant differences between months of allopurinol use or febuxostat use for MI, stroke or composite of MACE and all-cause mortality (Table 1). Conclusion: By using marginal structural model to reduce bias caused by non-random censoring and treatment assignment, we found no differences in the risk of MACE events between febuxostat and allopurinol users in a large cohort of veterans with gout.
To cite this abstract in AMA style:
Zembrzuska H, Gao Y, Girotra S, Lund B, Saag K, Curtis J, Vaughan-Sarrazin M, Singh N. Comparative Risk of Cardiovascular Events in US Veterans with Gout Treated with Febuxostat versus Allopurinol [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparative-risk-of-cardiovascular-events-in-us-veterans-with-gout-treated-with-febuxostat-versus-allopurinol/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-risk-of-cardiovascular-events-in-us-veterans-with-gout-treated-with-febuxostat-versus-allopurinol/