Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with rheumatoid arthritis (RA) can have various pulmonary comorbidities including asthma, chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD). Biologics can pose a potential risk for acute exacerbations (AE) of these pulmonary comorbidities by immunosuppression, drug-induced lung injury, or other mechanisms. We therefore compared the risk of pulmonary AE among RA patients initiating abatacept vs. TNF inhibitors (TNFi) with chronic pulmonary comorbidities at baseline.
Methods: We conducted a cohort study using claims data from Medicare (2008-2013) or a commercial health plan (MarketScan 2006-6/2015) in the US. Adults with RA and chronic pulmonary condition (i.e., asthma, COPD, or ILD) who initiated abatacept or TNFi were identified based on a combination of diagnosis codes and use of disease-specific medications. The cohort entry date was the 1st use of abatacept or TNFi after ≥12 month continuous enrollment. The primary outcome was a composite endpoint defined as any of: 1) inpatient stays or 2) ED visits for asthma, COPD, or ILD, or 3) outpatient visits for asthma, COPD or ILD plus dispensing of oral corticosteroids or antibiotics, or for respiratory complications such as pneumonitis and bronchitis. Secondary outcomes were individual components of the primary composite endpoint. Follow-up time started from the day after cohort entry to the earliest event of outcome occurrence, drug discontinuation, nursing home admission, disenrollment, end of study period, or death. To control for >50 potential confounders at baseline, abatacept initiators were matched to TNFi initiators on a propensity score (PS) with a 1:2 ratio in each of the three pulmonary condition subgroups. We estimated incidence rates (IR) and hazard ratios (HR) of the outcomes among abatacept initiators versus TNFi in the main cohort and the subgroups. PS-matched HRs from the two databases were then pooled using inverse variance-weighted fixed-effect method.
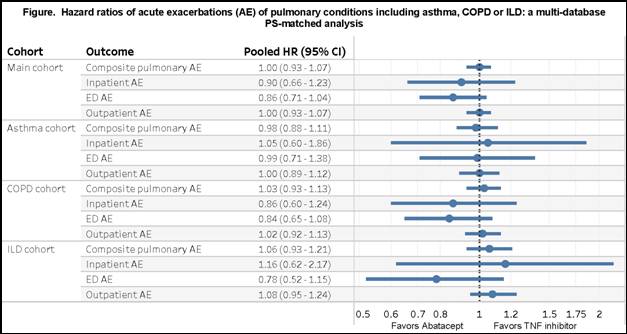
Results: After PS matching, we included 1,517 abatacept and 3,034 TNFi initiators with RA and chronic pulmonary condition from both databases. Mean (SD) age was 73.0 (6.1) years in Medicare and 61.2 (12.1) years in MarketScan. At baseline, 44% in MarketScan and 57% in Medicare used methotrexate. IR of the composite outcome per 100 person-years ranged from 47.6-49.6 in the abatacept group and from 51.8-53.5 in the TNFi group. For the primary composite outcome, the HR comparing abatacept to TNFi was 1.00 (95%CI 0.92-1.09) in Medicare and 1.00 (95%CI 0.89-1.12) in MarketScan with a pooled HR of 1.00 (95%CI 0.93-1.07). Secondary and subgroup analyses showed similar results (Figure).
Conclusion: Among patients with RA and chronic pulmonary condition, acute exacerbations of underlying asthma, COPD or ILD occurred frequently but we found no difference in the exacerbation risk between abatacept and TNFi initiators.
To cite this abstract in AMA style:
Kang EH, Jin Y, Dejene S, Brill G, Desai RJ, Sparks JA, Kim SC. Comparative Pulmonary Safety of Abatacept and Tumor Necrosis Factor Inhibitors in Patients with RA and Chronic Pulmonary Condition [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparative-pulmonary-safety-of-abatacept-and-tumor-necrosis-factor-inhibitors-in-patients-with-ra-and-chronic-pulmonary-condition/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-pulmonary-safety-of-abatacept-and-tumor-necrosis-factor-inhibitors-in-patients-with-ra-and-chronic-pulmonary-condition/