Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Network meta-analyses of phase 3 clinical trial data involving JAK inhibitor (JAKi)-treated patients with RA and an inadequate response to conventional synthetic DMARDs showed that upadacitinib (UPA) 15 mg had numerically higher efficacy vs other approved JAKis.1 However, real-world data on the effectiveness of UPA in clinical practice relative to other JAKis is limited. Here, we assessed the effectiveness of UPA vs other JAKis, including tofacitinib, baricitinib, and peficitinib, using real-world data.
Methods: Data were extracted from the Adelphi RA Disease-Specific Programme, a cross-sectional study with elements of retrospective data collection. Surveys were administered to rheumatologists and their patients in the EU, UK, Japan, Canada, and USA from July 2021 to February 2022. Patients receiving UPA 15 mg or other JAKis for ≥6 months were included. Physician-reported clinical outcomes included disease activity (with formal DAS28 scoring available for 35.5% of patients) categorized as follows: DAS28 remission (< 2.6) and low, (2.6–< 3.2), moderate (3.2–5.1), and high ( >5.1) disease activity (LDA, MDA, and HDA, respectively); pain and fatigue (none, mild, moderate, or severe); and medication adherence (completely adherent or not). A high degree of correlation (Spearman’s coefficient, 0.68) was observed between formal DAS28 scores and disease activity category, justifying the use of physician-reported DAS28 categories. Unadjusted physician-reported outcomes are descriptively reported as the change in disease activity from initiation of current treatment to most recent follow up at ≥6 months. Adjusted physician-reported outcomes were compared for UPA vs other JAKis at the most recent follow-up visit using inverse probability weighted regression adjustment (IPWRA) methods. Results are reported as predicted percentages along with P values for each treatment group.
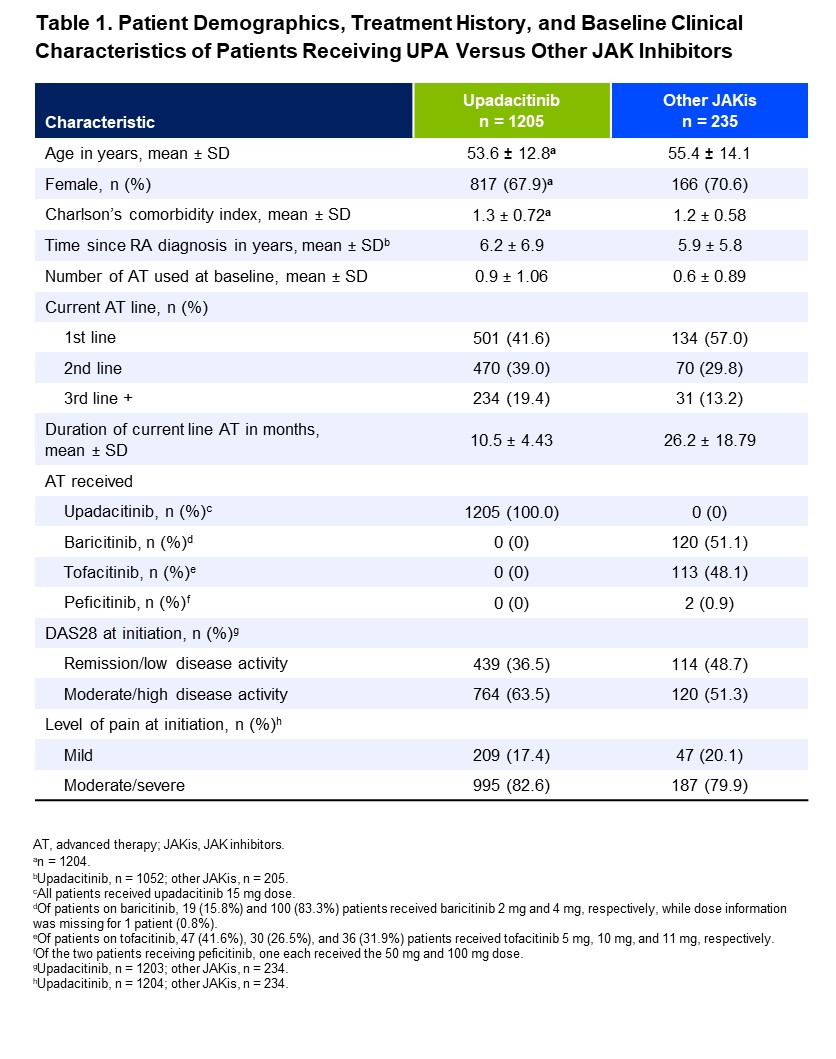
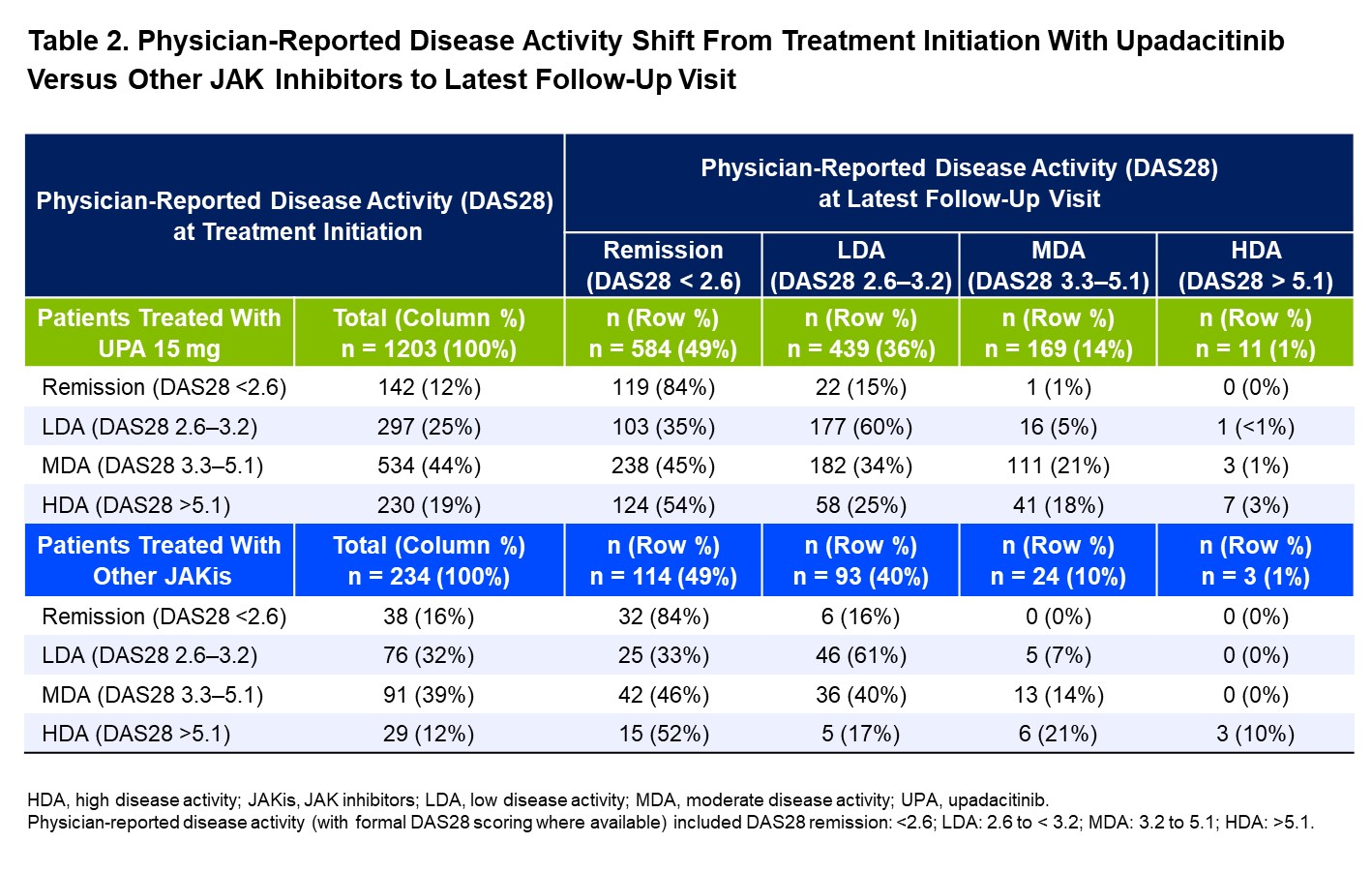
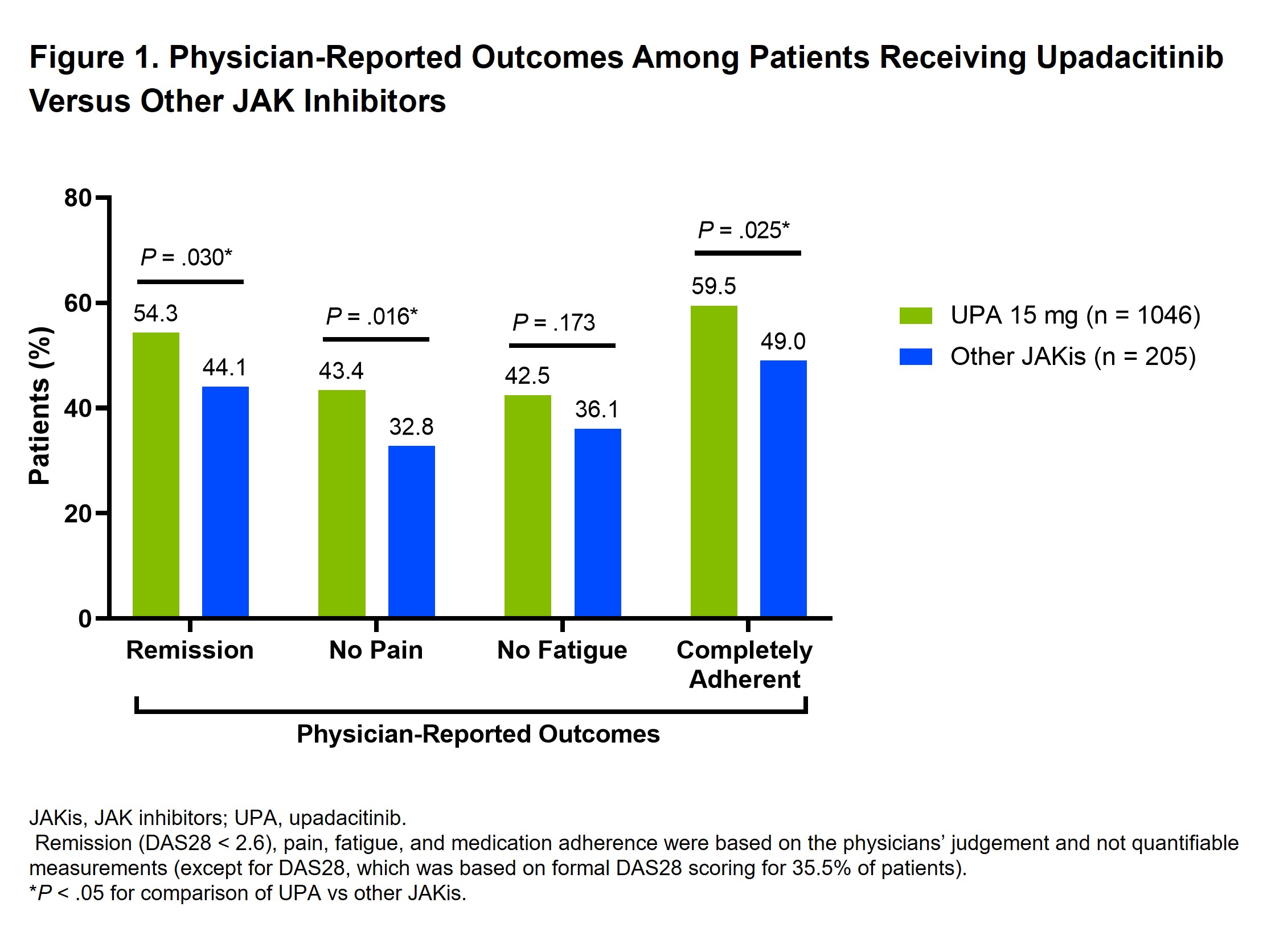
Results: A total of 1440 patients were included (UPA 15 mg, n=1205; other JAKis, n=235), with most patients in the other JAKis group receiving baricitinib (n=120) or tofacitinib (n=113) and the remainder receiving peficitinib (n=2). Baseline characteristics are shown in Table 1, and the two treatment groups were weighted based on multiple covariates. At treatment initiation, 63% of UPA-treated patients were in MDA/HDA, while 51% of other JAKi-treated patients were in MDA/HDA. At the most recent follow-up visit, 85% and 88% of patients receiving UPA and other JAKis, respectively, were in LDA/remission (Table 2). IPWRA showed that patients on UPA were significantly more likely to have achieved physician-reported DAS28 remission (54% vs 44%, P=.03), no pain (43% vs 33%, P=.02), and complete medication adherence (60% vs 49%, P=.03) compared to those receiving other JAKis (Figure 1). Additionally, 43% of patients receiving UPA were evaluated as having no fatigue compared to 36% of patients receiving other JAKis (P=.17).
Conclusion: The findings of this real-world study of patients with RA demonstrate that greater proportions of patients attained physician-reported DAS28 remission, absence of pain, and medication adherence with UPA vs other JAKis.
References
1. Pope J, et al. Adv Ther 2020;37:2356-72.
To cite this abstract in AMA style:
Taylor P, Kadakia A, Milligan J, Strengholt S, Howell O, Patel P, Barlow S, Caporali R. Comparative Effectiveness of Upadacitinib versus Other JAK Inhibitors in Patients with Rheumatoid Arthritis in a Global Real-World Setting [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparative-effectiveness-of-upadacitinib-versus-other-jak-inhibitors-in-patients-with-rheumatoid-arthritis-in-a-global-real-world-setting/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-upadacitinib-versus-other-jak-inhibitors-in-patients-with-rheumatoid-arthritis-in-a-global-real-world-setting/