Session Information
Date: Monday, November 9, 2020
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Studies comparing the effectiveness of biologics in the treatment of axial spondyloarthritis (axSpA) are sparse [1]. One study comparing secukinumab and adalimumab biosimilar is ongoing. Secukinumab is an agent targetting Il-17. The aim of our analysis is to compare the effectiveness of the most frequently used biologic in axSpA since the marketing of secukinumab in all, first and second and third intention or more. Results are presented here.
Methods: Patients diagnosed with axSpA enrolled in RHUMADATA® giving informed consent and treated with secukinumab (SEC), adalimumab (ADA) or other TNF inhibitors (TNFi) on or after January 1, 2016 were extracted from the RHUMADATA® clinical database and registry. RHUMADATA® provides socio-demographics, comorbidities, patient-reported outcomes, disease activity indices, laboratory variables, rheumatologist exam report data, use of concomitant medications and axSpA treatment and reasons for treatment cessation. Kaplan-Meier survival curves and proportional hazard models were used to analyze time to treatment cessation.
Results: A total of 383 patients with axSpA were extracted from the RHUMADATA® registry. Seventy-four were treated with SEC, 96 with ADA and 213 with other TNFi (certolizumab, etanercept, golimumab or infliximab). Overall, 47.3% were women, the average age and disease duration at treatment initiation were respectively 44.8 (±standard deviation (STD)=13.3) and 6.0 (8.4) years. The overall age adjusted Charlson Comorbidity Index (aCCI) was 1.3 (2.1). The proportion of patients diagnosed and/or treated for diabetes and hypertension respectively were 14.9 and 40.5% (SEC), 5.2 and 25.0% (ADA) and 12.7 and 30.5% (TNFi). Overall BASDAI and BASFI scores were 6.0 (2.3) and 5.0 (2.6). SEC, ADA and TNFi were used in first intention in 18.9, 61.5 and 41.3% of patients respectively. Kaplan-Meier logrank p-values were 0.0112, 0.0689, and 0.3479 when comparing the three groups in all, first and second and third or more intentions. Pairwise comparisons showed better retention for ADA treated patients in all and first and second intentions (early treatments). A Cox proportional hazard model adjusting for age at diagnosis, disease duration at treatment initiation, aCCI, the number of prior advanced treatments and concomitant use of methotrexate at treatment initiation demonstrated a statistically better retention of ADA over TNFi.
Conclusion: Based on our real-world datasets, ADA had better retention than SEC in treating early axSpA.
References:
Maksymowych WP, Strand V, Nash P, et al. Comparative effectiveness of secukinumab and adalimumab in ankylosing spondylitis as assessed by matching-adjusted indirect comparison. Eur J Rheumatol. 2018;5(4):216-223. doi:10.5152/eurjrheum.2018.18162
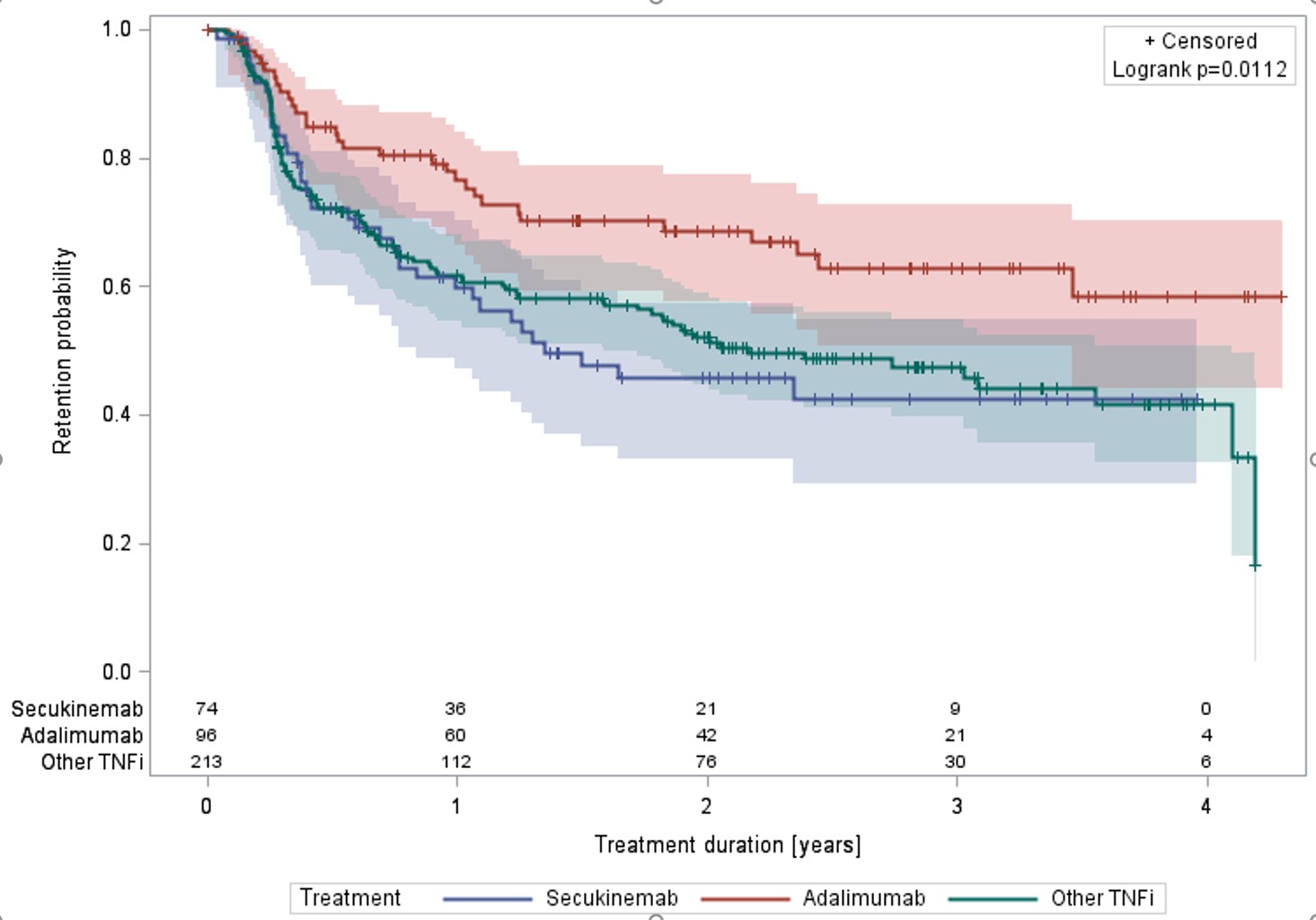 Retention of ADA, SEC and TNFi – all intentions
Retention of ADA, SEC and TNFi – all intentions
 Retention of ADA, SEC and TNFi – first and second intentions
Retention of ADA, SEC and TNFi – first and second intentions
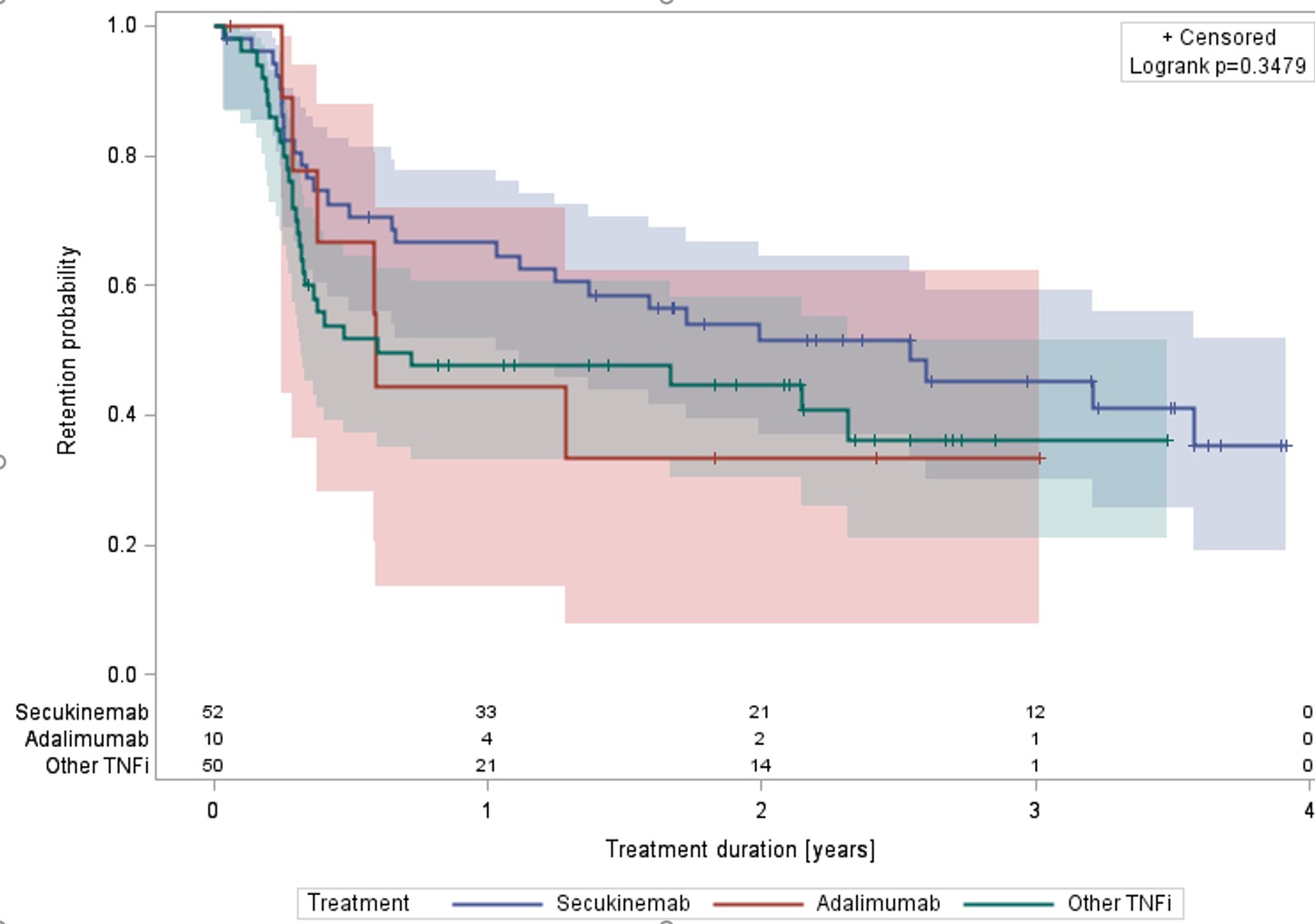 Retention of ADA, SEC and TNFi – third + intentions
Retention of ADA, SEC and TNFi – third + intentions
To cite this abstract in AMA style:
Choquette Sauvageau L, Choquette D, Bessette L, Ferdinand I, Haraoui B, Massicotte F, Pelletier J, Raynauld J, Rémillard M, Sauvageau D, Villeneuve �, Coupal L. Comparative Effectiveness of Secukinumab, Adalimumab and Other Tumor Necrosis Factor Inhibitors in the Treatment of Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparative-effectiveness-of-secukinumab-adalimumab-and-other-tumor-necrosis-factor-inhibitors-in-the-treatment-of-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-secukinumab-adalimumab-and-other-tumor-necrosis-factor-inhibitors-in-the-treatment-of-axial-spondyloarthritis/
