Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: Osteoporosis and Metabolic Bone Disease – Basic and Clinical Science
Session Type: Abstract Session
Session Time: 8:00AM-9:00AM
Background/Purpose: Although clinical trials evaluating the efficacy of osteoporosis (OP) therapies have shown that denosumab (Dmab) significantly increases bone mineral density at pivotal sites compared with oral bisphosphonates, there is little evidence from head-to-head randomized trials or observational studies evaluating fracture outcomes. This study evaluated the real-world effectiveness of Dmab compared with alendronate (Aln) in reducing fracture risk among commercially-insured postmenopausal women in the U.S. treated for OP.
Methods: Women 55 years or older who newly initiated Dmab or Aln from 2012 to 2019 were identified using an administrative health claims database consisting of members of large commercial and Medicare Advantage plans. Using a doubly-robust inverse-probability of treatment and censoring weight function, we estimated the risk ratio (RR) and 95% confidence intervals (CI) of major osteoporotic, hip, clinical vertebral, and non-vertebral fractures over a 24-month follow-up period among treatment-experienced patients (switched from a different OP therapy within 455 days of treatment initiation). Because Dmab is primarily used as a second-line therapy, and newly approved therapies are typically administered to patients with more severe disease or among those with limited treatment options, numerous prespecified sensitivity and subgroup analyses were conducted in patients at higher fracture risk and among those initiating treatment in different time periods (ie, after Oct 2015) following Dmab approval.
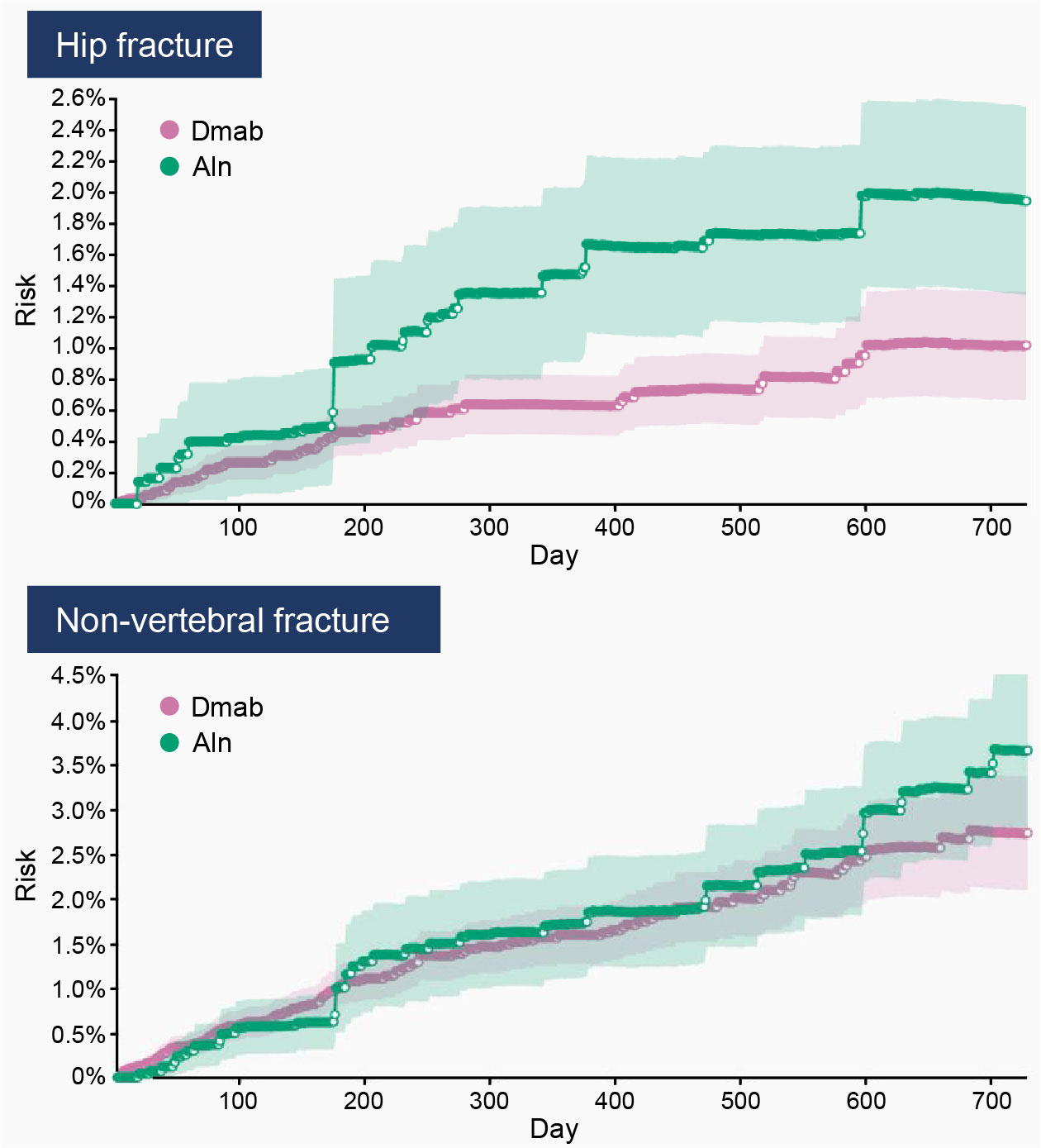
Results: When comparing Dmab (n=13,871) with Aln (n=8,747) in patients over the entire study period (2012–2019), no differences in the incidence of fracture outcomes were observed. However, among patients initiating treatment in 2015 or later (ie, recent data cohort), Dmab users had a 48% reduction in hip (RR=0.52, 95% CI: 0.28–0.77) and a 25% reduction in non-vertebral (RR=0.75; 95% CI: 0.50–0.99) fracture risk compared with Aln users. Cumulative incidence curves within the recent data cohort suggest a growing difference in the risk of hip and non-vertebral fractures through 24 months of treatment when patients adhere to Dmab compared with Aln (Fig. 1).
Conclusion: In this cohort of postmenopausal women receiving OP treatment in clinical practice, we observed a clinically meaningful reduction in fractures for patients who remained adherent to Dmab compared with Aln through 24 months, once the effects of preferential prescribing of Dmab to high-risk patients in the early years after drug approval were removed. These findings need to be confirmed in larger studies using recent data and longer term follow up.
To cite this abstract in AMA style:
Kim M, McGrath L, Pritchard D, Samai P, Lin J, Stad R, Spangler L, McDermott M, Bradbury B, Brookhart M. Comparative Effectiveness of Osteoporosis (OP) Therapies Among a Population of Postmenopausal (PM) Women in the United States (U.S.) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/comparative-effectiveness-of-osteoporosis-op-therapies-among-a-population-of-postmenopausal-pm-women-in-the-united-states-u-s/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-osteoporosis-op-therapies-among-a-population-of-postmenopausal-pm-women-in-the-united-states-u-s/