Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Sarilumab, an IL-6 receptor inhibitor, and upadacitinib, a Janus kinase (JAK) 1 inhibitor, are both approved for the treatment of patients with moderately to severely active RA. In the absence of head-to-head trials, it has been suggested that treatments can be indirectly compared using the matching-adjusted indirect comparison (MAIC) method, in which patient-level data from one trial can be weighted for preselected patient characteristics to match against aggregated data from a comparator trial. Here, we report an MAIC comparison of sarilumab and upadacitinib outcomes using data from 2 phase 3 trials in patients with RA who were refractive to previous treatment with biologic DMARDs, including TNF inhibitors.
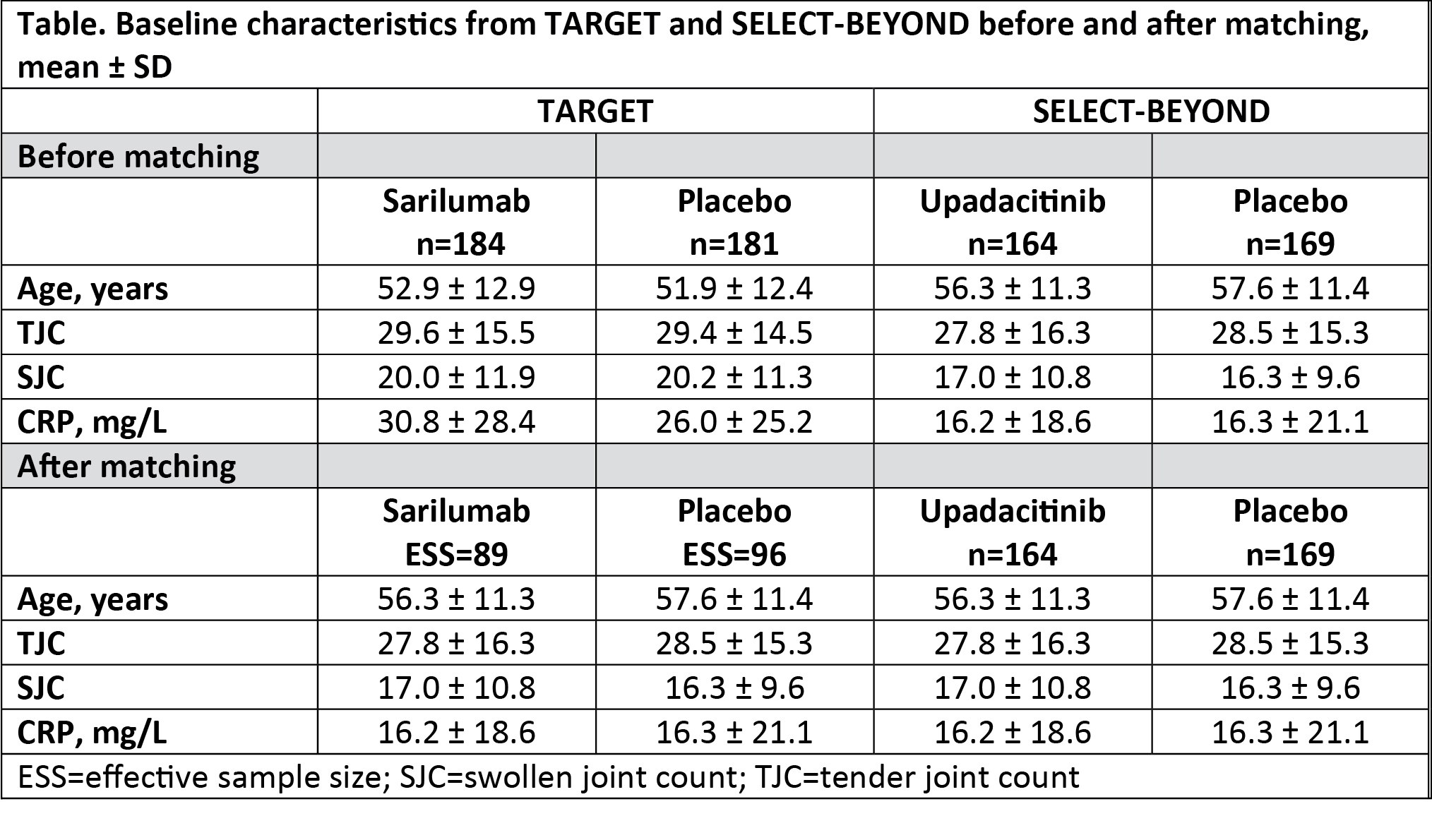
Methods: TARGET (NCT01709578) and SELECT-BEYOND (NCT02706847) were randomized, placebo-controlled trials, and included adults with active RA who had received synthetic DMARDs and had an inadequate response or intolerance to ≥1 biologic DMARD. Patient-level data of participants from TARGET treated with subcutaneous placebo or sarilumab (200 mg once every 2 weeks), and aggregated data of patients from SELECT-BEYOND treated with placebo or oral upadacitinib (15 mg/day) were analyzed. Baseline characteristics with notable differences between the 2 trials that were potential treatment effect modifiers were identified. They included age, tender joint count (TJC) of 68 counts, swollen joint count (SJC) of 68 counts, and CRP concentration. Individual patients from the TARGET trial were assigned weights such that weighted mean baseline characteristics of the treatment effect modifiers matched those from SELECT-BEYOND. Treatment effects for the adjusted TARGET population at Week 12 were compared with published aggregate data from SELECT-BEYOND. Endpoints evaluated included the proportions of patients achieving ACR20, ACR50, ACR70, DAS28-CRP < 3.2 (low disease activity), and DAS28-CRP < 2.6 (remission).
Results: The analysis included 365 patients from TARGET (sarilumab, n=184; placebo, n=181) and 333 patients from SELECT-BEYOND (upadacitinib, n=164; placebo, n=169). After weighting, the 2 trials were matched for the treatment effect modifiers, which resulted in the decline of the effective sample size of TARGET population to 185 (sarilumab, n=89; placebo, n=96) (Table). After MAIC, the odds of achieving various clinical outcomes versus placebo were similar for sarilumab and upadacitinib for all evaluated clinical outcomes (all nominal p >0.05; Figure). A nonadjusted analysis yielded similar results (data not shown).
Conclusion: In this MAIC analysis of patients from the TARGET and SELECT-BEYOND trials matched for several key baseline characteristics, efficacy of sarilumab was statistically similar to upadacitinib when comparing ACR20/50/70 and DAS28-CRP low disease activity/remission outcomes in patients with active RA who had an inadequate response to biologic DMARDs. Prospective head-to-head comparisons are needed to confirm these findings.
To cite this abstract in AMA style:
Huizinga T, Choy E, Praestgaard A, van Hoogstraten H, LaFontaine P, Guyot P, Aletaha D, Müller-Ladner U, Tanaka Y, Curtis J, Fleischmann R. Comparative Clinical Efficacy of Sarilumab versus Upadacitinib over 12 Weeks: Matching-Adjusted Indirect Comparison Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparative-clinical-efficacy-of-sarilumab-versus-upadacitinib-over-12-weeks-matching-adjusted-indirect-comparison-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-clinical-efficacy-of-sarilumab-versus-upadacitinib-over-12-weeks-matching-adjusted-indirect-comparison-analysis/