Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: JAK inhibitors (JAKi) and biologic disease-modifying antirheumatic drugs (bDMARDs) have proven efficacy in the treatment of rheumatoid arthritis (RA). However, their potential for increasing the risk of malignancy in Asian remained unclear. The aim of this study was to investigate the incidence and risk factors associated with malignancies among Asian patients with RA who are receiving conventional synthetic DMARDs (csDMARDs), bDMARDs, and JAKi .
Methods: Between January 2006 and July 2021, 3,804 patients with RA were retrospectively evaluated. Patients with missing data or a history of prior cancer diagnosis were excluded. bDMARDs included TNF inhibitors (TNFi) (etanercept, adalimumab, golimumab, and certolizumab pegol), anti-IL-6 receptor monoclonal antibody (tocilizumab), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)-immunoglobulin (Ig) fusion protein (abatacept), and B-cell-depleting agents (anti-CD20 monoclonal antibody, rituximab). JAKi included tofacitinib, baricitinib, and upadacitinib.
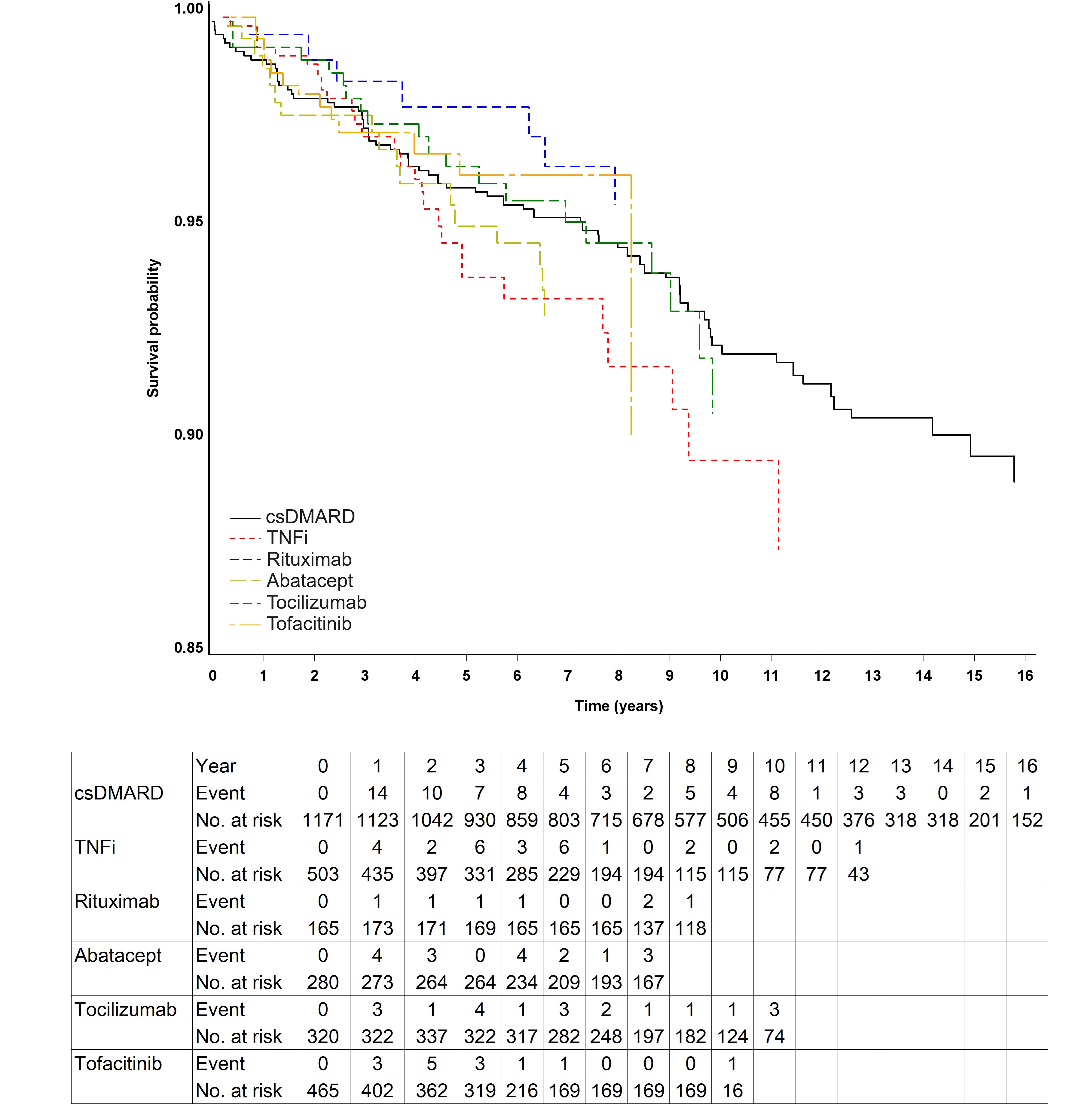
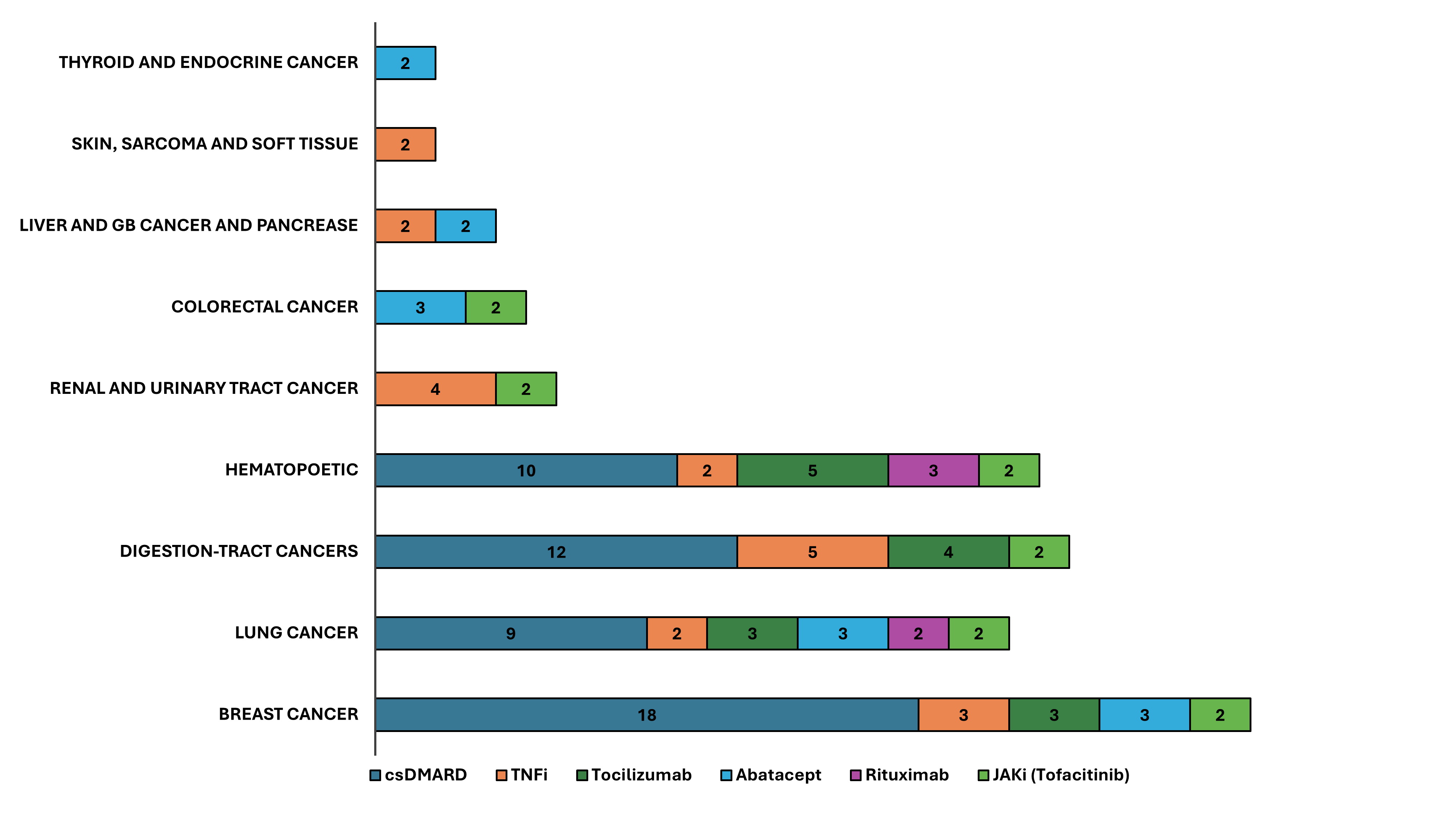
Results: During the 20,759 patient-years (PYs) of follow-up, a total of 161 of 2,904 patients (5.54 %) developed cancer, resulting in a crude incidence of malignancy of 7.76 events per 1000 PYs. Patients treated with TNFi exhibited the highest risk of developing malignancie (10.13 per 1000 PYs), followed by abatacept, tocilizumab, csDMARDs, JAKi, and rituximab group (8.80, 7.77, 7.59, 6.91, and 4.77 per 1000 PYs, respectively) (Figure 1). Among the 161 patients with malignancy events, breast cancer was the most common (N = 29), followed by gastrointestinal tract cancer (N = 24), hematopoietic malignancies (N = 23), lung cancer (N = 21), colorectal cancer (N = 15) and renal/urinary tract cancer (N = 13) (Figure 2). The rituximab group had a significantly lower hazard of malignancies compared with the TNFi group (adjusted HR: 0.29, 95% CI: 0.11-0.81, p = 0.018). While older patients (aged over 65 years) undergoing tofacitinib treatment showed a seemingly higher hazard of overall malignancies compared to those receiving TNFi, statistical significance was not achieved (adjusted HR: 2.29, 95% CI: 0.52-10.14, p = 0.218). In a multivariable Cox regression model, it showed that only an age at the diagnosis of RA greater than 65 years was an independent risk factor for the development of malignancy (adjusted HR: 1.63. 95% CI: 1.16-2.36, p = 0.007).
Conclusion: The results indicate that the risk of malignancies did not exhibit statistically significant differences among Asian RA patients treated with csDMARD-only, JAKi, and other bDMARD regimens, except for a potential association of rituximab with a lower incidence. However, it’s important to note that regular cancer screenings are still advisable for these patients while they are undergoing JAKi/biologic treatment.
To cite this abstract in AMA style:
Chen M, Chen M, Chang S, CHEN D. Comparable Malignancy Risk of Janus Kinase Inhibitors with Conventional Synthetic and Biologic DMARDs in Asian Patients with Rheumatoid Arthritis: A Comprehensive Analysis from Two Medical Centers [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparable-malignancy-risk-of-janus-kinase-inhibitors-with-conventional-synthetic-and-biologic-dmards-in-asian-patients-with-rheumatoid-arthritis-a-comprehensive-analysis-from-two-medical-centers/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparable-malignancy-risk-of-janus-kinase-inhibitors-with-conventional-synthetic-and-biologic-dmards-in-asian-patients-with-rheumatoid-arthritis-a-comprehensive-analysis-from-two-medical-centers/