Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: 30+ million US adults have OAKP, (13% women / 10% men). After years of using NSAIDs, analgesics, surgery and IAI, many patients remain with OAKP. CNTX is a 1 mg dose of capsaicin for IAI to reduce OAKP.
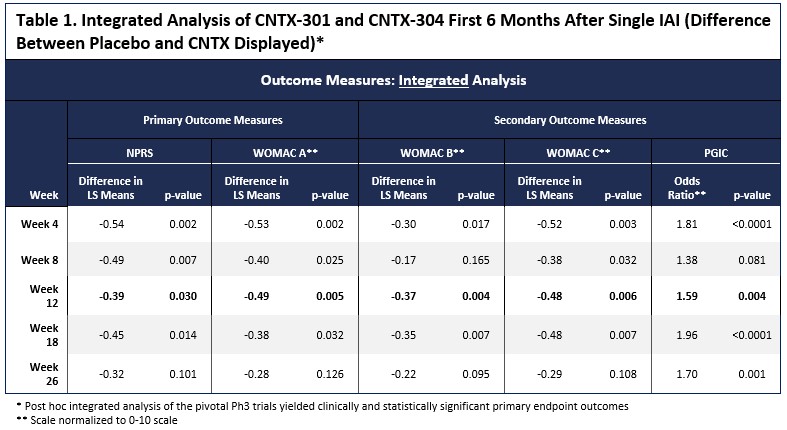
Methods: The phase 3 trials powering was based on the phase 2 trial showing a standard effect size (SES) of 0.55. Although each phase 3 trial (OAKP 301 and 304) was powered at >95% on the primary outcome measure, to allow for sufficient power for the key secondary outcome measures. The phase 3 trials were likely underpowered. The two nearly identical phase 3 trials did not meet the primary endpoint but nevertheless showed numerical superiority of CNTX over placebo. Given that the trials were nearly identical (except for a second injection in the OA-304), the results were combined for an integrated analysis. The analysis assessed the primary time point at Week 12 with the numeric pain rating scale (Primary OA-301) between (NPRS [0 – 10; 0 = no pain, 10 worst pain possible]) and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) A (Primary OA-304) (pain subscale; [0-50] -lower values have greater pain), WOMAC B (Knee stiffness [0-20]), WOMAC C (Function; [0-170]) and Patient Global Assessment of Change (PGIC;[0-7]).
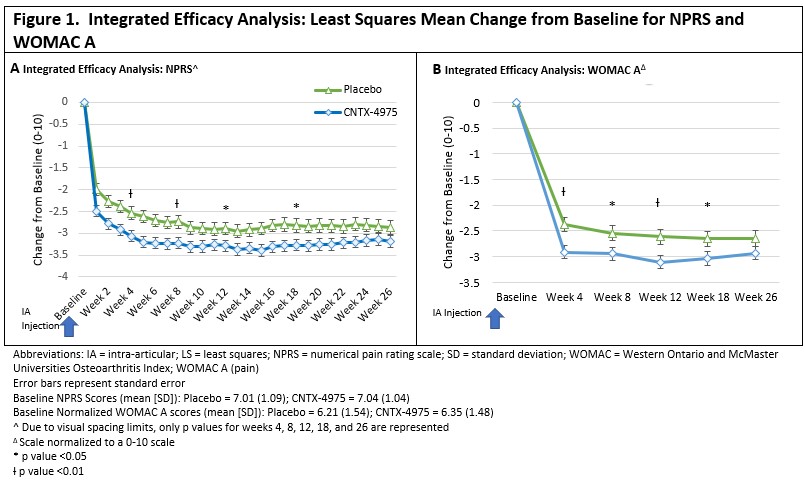
Results: Between the two trials, 650 subjects received 1 dose of study drug (276 placebo, 373 CNTX). The analysis of the primary and key secondary endpoints was conducted. With the combined data sets, CNTX-separated from placebo at all time points for 6 months after a single injection. (Figure 1). The Week 12 primary endpoint reached statistical significance across all endpoints (NPRS P=0.030, WOMAC A P = 0.005, WOMAC B P=0.004, WOMAC C P=0.006 and PGIC P=0.004), as shown in Table 1 and Figure 1.Significant differences between placebo and CNTX were also evident at multiple other timepoints through Week 26.
Overall safety was acceptable with CNTX. Knee radiographs from Baseline to Week 52 showed no difference in KOA progression between placebo and CNTX in both trials, with no rapidly progressive osteoarthritis.
Conclusion: The separate Phase 3 trials did not meet their primary endpoints, suggesting that each trial by itself was underpowered. Combining the two trials demonstrated statistical separation and clinically meaningful benefit, and safety was acceptable. The safety database satisfies the International Conference on Harmonization (ICH) guideline for safety assessments.
To cite this abstract in AMA style:
Connolly J, Campbell J, Stevens R, Newman C. Combined Datasets for CNTX-4975 Osteoarthritis Knee Pain (OAKP) Phase 3 Trials (OAKP 301 and 304) to Assess Overall Efficacy and Safety of an Intra-articular Injection (IAI) of CNTX-4975-05 (CNTX) in Subjects with Chronic, Moderate-to-severe Osteoarthritis Knee Pain (MSOAKP) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/combined-datasets-for-cntx-4975-osteoarthritis-knee-pain-oakp-phase-3-trials-oakp-301-and-304-to-assess-overall-efficacy-and-safety-of-an-intra-articular-injection-iai-of-cntx-4975-05-cntx-in/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combined-datasets-for-cntx-4975-osteoarthritis-knee-pain-oakp-phase-3-trials-oakp-301-and-304-to-assess-overall-efficacy-and-safety-of-an-intra-articular-injection-iai-of-cntx-4975-05-cntx-in/