Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Systemic Sclerosis and Related Disorders – Clinical I: Trials and Therapeutics
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Scleroderma Lung Study (SLS) II established mycophenolate mofetil (MMF) as an active therapy for scleroderma-related interstitial lung disease (SSc-ILD) and the need to consider background MMF in trial designs. SLS III envisioned an upfront combination therapy in which the rapid onset and anti-fibrotic effects of pirfenidone (PFD) would complement the delayed immunosuppressive and anti-inflammatory effects of MMF.
Methods: An investigator-initiated multi-center, double-blind, placebo (PLA)-controlled Phase II trial evaluated combination therapy (MMF+PFD vs MMF+PLA) in those with SSc-ILD. Key inclusion criteria were age≥18, SSc-ILD with symptoms (Mahler dyspnea index), FVC% ≤ 85% predicted, disease duration ≤7 yrs, and any ground glass opacity on HRCT. Subjects were randomized to each arm, stratified by site and prior MMF therapy (naïve, >0 to ≤3 months, and >3 months to 6 months) with a planned enrollment of 150. The primary endpoint was the change from baseline in the mean forced vital capacity % predicted (FVC%) over the course of the 18-month treatment period. Treatment differences were tested using linear mixed effects models for longitudinal endpoints and ANCOVA for HRCT endpoints measured at screening and 18 months, both adjusting for prior MMF therapy and efficacy measure at screening or baseline.
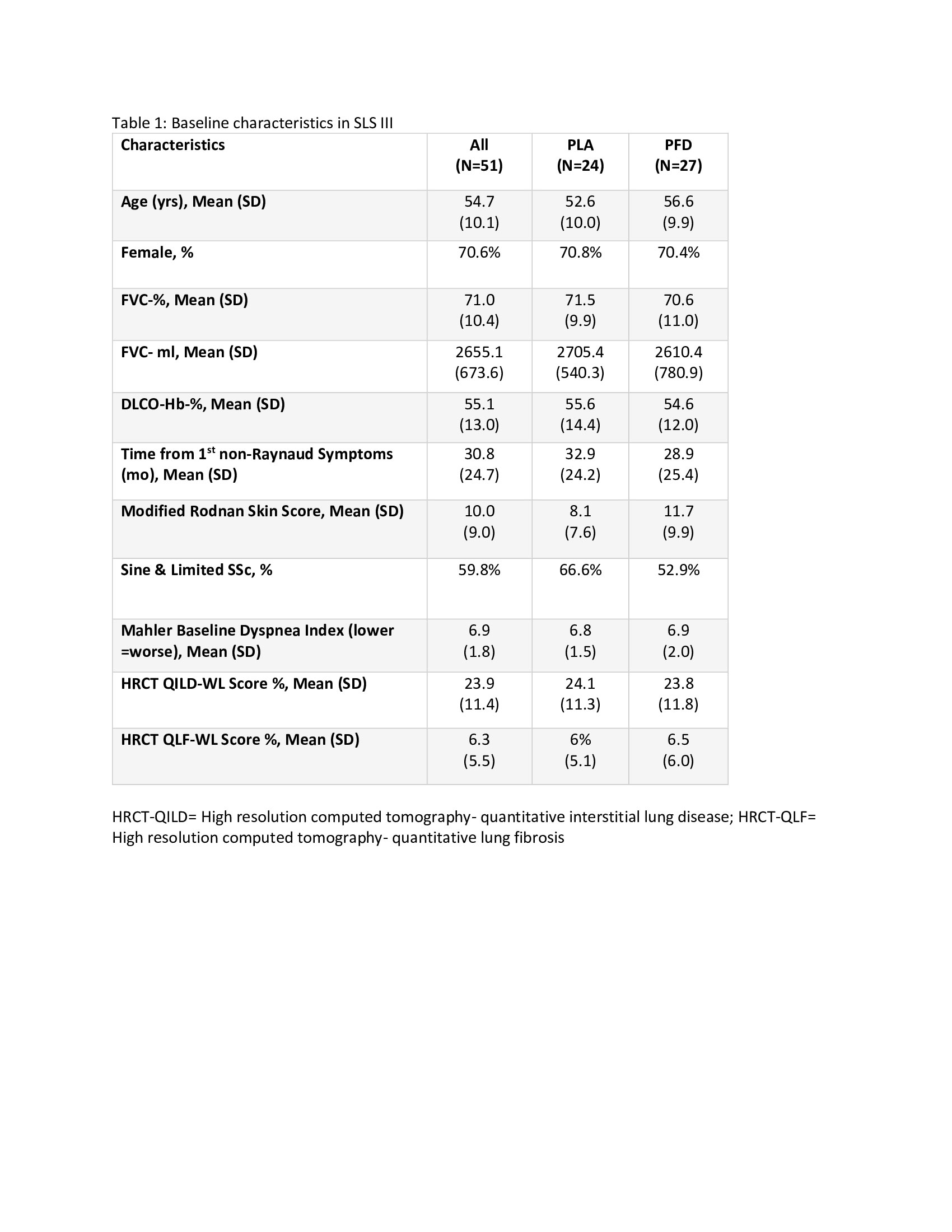
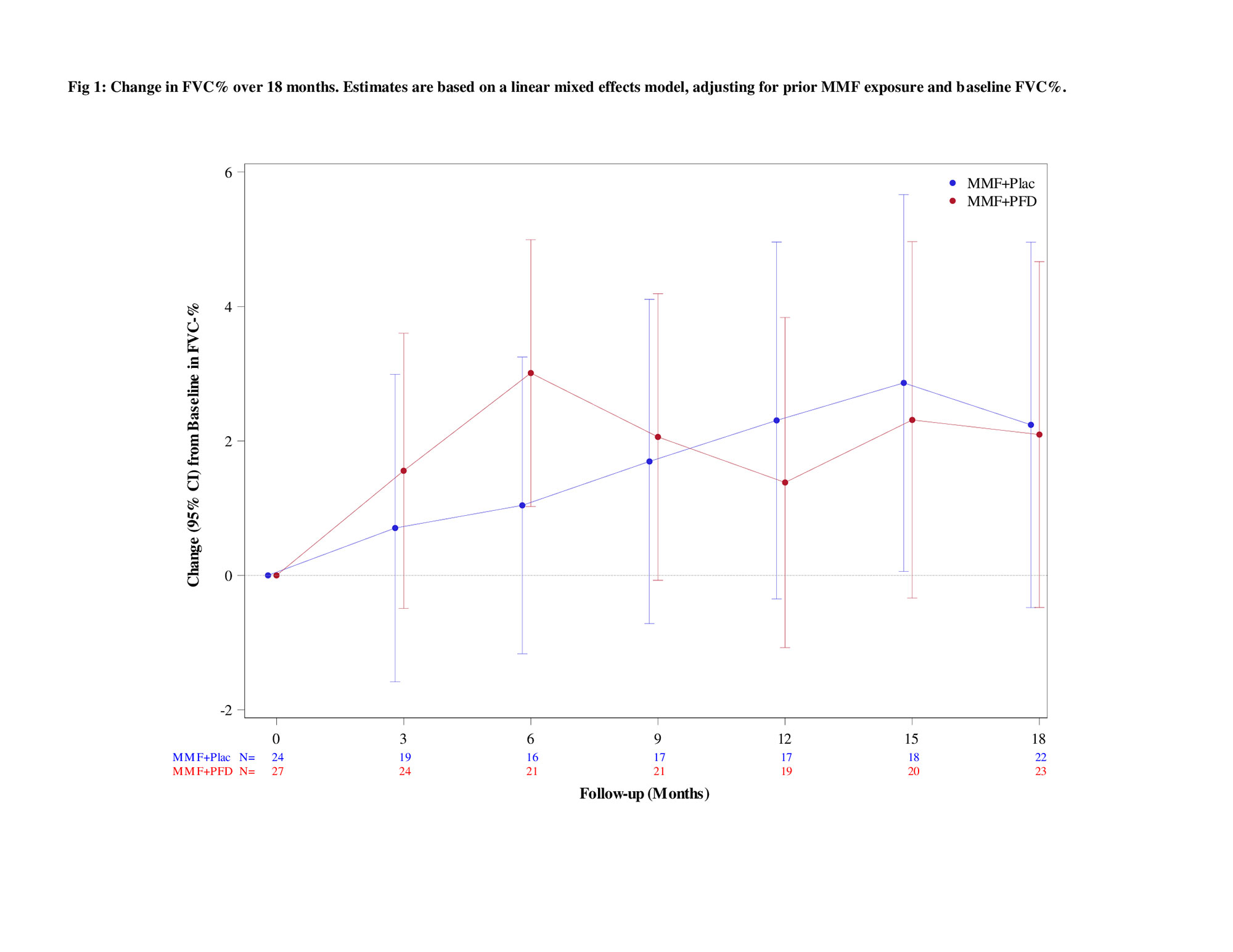
Results: 51 participants were randomized (76% naive; 14% ≤3 months MMF; 10% >3-6 months MMF) with 27 to MMF+PFD and 24 to MMF+PLA. Recruitment was prematurely stopped due to COVID-19 and the impact of prior drug treatment on eligibility. Table 1 details baseline characteristics. Overall, a similar magnitude of improvement in FVC% over 18 months occurred in both arms (2.24% MMF+PLA vs. 2.09% MMF+PFD; P=0.93) with a more rapid improvement noted in the MMF+PFD arm over 6 months (Figure 1). In addition, there were trends favoring the PFD arm in HRCT quantification of fibrosis (QLF) and interstitial lung disease (QILD) as well as in HAQ-DI, SGRQ-activity scale, and PROMIS-29 physical function scales, all meeting/exceeding minimally important differences (Table 2). 74.1% of subjects on MMF+PFD vs 29.2% on MMF+PLA had adverse events of special interest; the difference primarily related to gastrointestinal disorders (55.6% vs. 20.8%) and photosensitivity (14.8% vs 0%). 7 participants prematurely discontinued one or both study drugs, all in the MMF+PFD arm. There were no deaths.
Conclusion: Consistent with a primary hypothesis, upfront combination therapy (MMF+PFD) led to more rapid improvement in the FVC% (0-6 months) but with a similar overall improvement over 18 months as compared to MMF +PLA. Changes in HRCT outcomes and patient-reported outcomes tended to favor MMF+PFD. Side effects were similar to the published literature and more common with MMF+ PFD. Although the study was underpowered, it raises the question whether upfront MMF+PFD can lead to a more rapid improvement in FVC and greater impact on HRCT and patient-reported outcomes.
To cite this abstract in AMA style:
Khanna D, Spino C, Bernstein E, Goldin J, Tashkin D, roth M, SLS III Investigators O. Combination Therapy of Mycophenolate Mofetil and Pirfenidone vs. Mycophenolate Alone: Results from the Scleroderma Lung Study III [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/combination-therapy-of-mycophenolate-mofetil-and-pirfenidone-vs-mycophenolate-alone-results-from-the-scleroderma-lung-study-iii/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combination-therapy-of-mycophenolate-mofetil-and-pirfenidone-vs-mycophenolate-alone-results-from-the-scleroderma-lung-study-iii/