Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite advances in psoriatic arthritis (PsA) treatment, remission remains elusive for many patients. This has prompted growing interest in combining biologic and targeted synthetic DMARDs (b/tsDMARDs). However, real-world data on such combinations are limited. This case series evaluates clinical outcomes and safety of b/tsDMARD combinations in PsA patients.
Methods: Case series of PsA patients receiving combined b/tsDMARD therapy, including TNFi, JAKi, TYK2i, IL17i, IL23i, IL-12/23i for effectiveness and safety. Combinations with Apremilast (APR) were analyzed for safety. Clinical and safety data were prospectively collected using standardized instruments within a longitudinal PsA cohort, and supplemented via chart review. Effectiveness was assessed by changes in joint counts, DAPSA, PASI, BSA, and patient-reported outcomes (NRS for pain, skin, and global) at 3-6 and 6-12 months. Safety outcomes included infections and non-infectious adverse events (AEs).
Results: We identified 22 patients treated with bDMARDs + JAKi or TYK2i, some with multiple regimens. Eleven (50%) were female. Complete information on PsA measure of activity was available for 9 patients (Table 1), while 13 had partial data, including age, sex, skin activity, and AEs. Numerical improvements were observed across disease activity measures, with Figure 1 showing patients with visits in the prespecified timeframes, and Table 2 showing the safety data.In the bDMARD + JAKi group (n=6), IL17i + JAKi were the most frequent combinations (n=4), used for 4,145 days (11.35 patient-years [PY]). One case of mild infectious stomatitis was reported, but treatment was not discontinued. IL23i + JAKi (n=2), used for 1,337 days (3.7 PY), had no AEs. For the bDMARD + TYK2i group (n=19), IL-17i + TYK2i (n=9) were used for 3,229 days (8.5 PY). One patient experienced two mild upper respiratory infections (URIs) on bimekizumab and deucravacitinib, prompting a switch for risankizumab with deucravacitinib. IL-23i with TYK2i (n=10) were used for 3,325 days (9.1 PY), with two cases of mild URIs leading to a switch to bimekizumab monotherapy, and one case of folliculitis, where therapy was continued. One patient received TNFi + TYK2i for 324 days (0.9 PY), with no AEs. We identified 16 patients on bDMARDs + APR (total of 22 combinations, with a median duration of 825 days), including IL12/23i or IL23i + APR (8 combinations, duration: 360–2,250 days), IL17i + APR (8 combinations, duration: 90–2,790 days), TNFi + APR (5 combinations, duration: 180–2,340 days), and JAKi + APR (1 combination, 540 days). Two cases of diarrhea were observed, but no infections occurred.
Conclusion: Overall, the safety profile of bDMARD combinations with tsDMARDs was favorable. Infections, primarily URIs, were the most common AEs – mild in severity, non-hospitalized, and rarely requiring treatment change. Short-term improvements were observed in both musculoskeletal and skin domains. Our findings, drawn from a prospective cohort with standardized data collection, support the effectiveness and safety of combination therapy in selected PsA cases. Randomized controlled trials are needed to confirm long-term efficacy and safety.
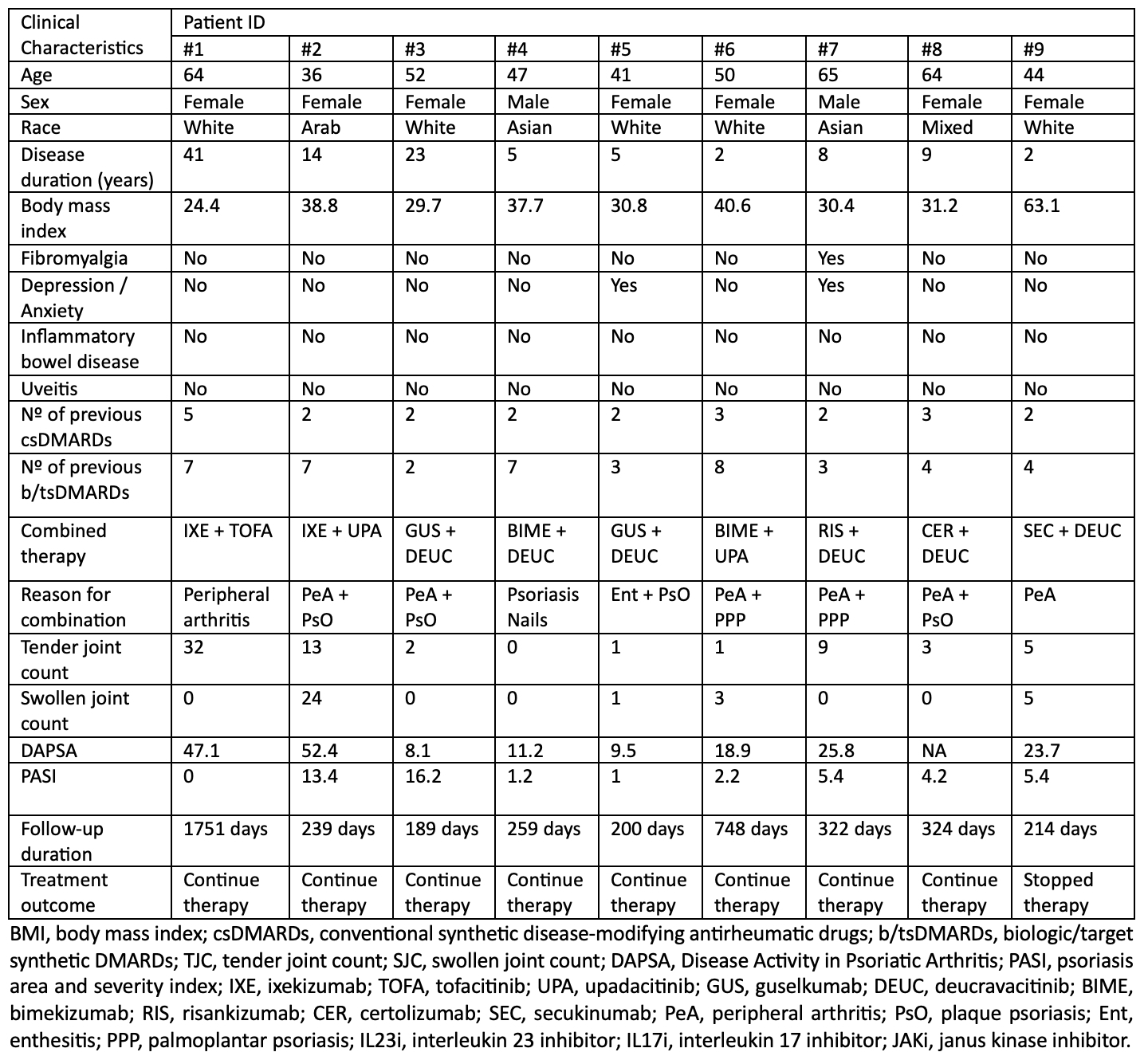 Table 1. Demographics and clinical characteristics of patients on combined bDMARD and JAK/TYK2i.
Table 1. Demographics and clinical characteristics of patients on combined bDMARD and JAK/TYK2i.
.jpg) Table 2. Safety analysis. IL17i, interleukin 17 inhibitor; tsDMARD, target-synthetic disease-modifying antirheumatic drug; IL23i, interleukin 23 inhibitor; TNFi, tumor necrosis factor inhibitor; IL12/23i, interleukin 12/23 inhibitor; JAKi, janus kinase inhibitor.
Table 2. Safety analysis. IL17i, interleukin 17 inhibitor; tsDMARD, target-synthetic disease-modifying antirheumatic drug; IL23i, interleukin 23 inhibitor; TNFi, tumor necrosis factor inhibitor; IL12/23i, interleukin 12/23 inhibitor; JAKi, janus kinase inhibitor.
.jpg) Figure 1. Individual trajectories after starting combined therapy for patients with follow-ups according to protocol.
Figure 1. Individual trajectories after starting combined therapy for patients with follow-ups according to protocol.
To cite this abstract in AMA style:
Lucas Ribeiro A, Carrizo Abarza V, Yeung J, Maliyar K, Sood S, Bagit A, Sachdeva M, Koppikar S, Gladman D, Chandran V, Eder L. Combination of Biological and Targeted Synthetic Disease-Modifying Antirheumatic Drugs in Psoriatic Arthritis: A Case-Series [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/combination-of-biological-and-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-psoriatic-arthritis-a-case-series/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combination-of-biological-and-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-psoriatic-arthritis-a-case-series/
