Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a complex inflammatory disease where achieving remission remains challenging despite multiple approved biologic DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs). The low remission rates have spurred interest in combining b/tsDMARDs. Given the paucity of data on such combinations, this case series aims to describe the outcomes of combined b/tsDMARD therapy, providing insights into its effectiveness and safety.
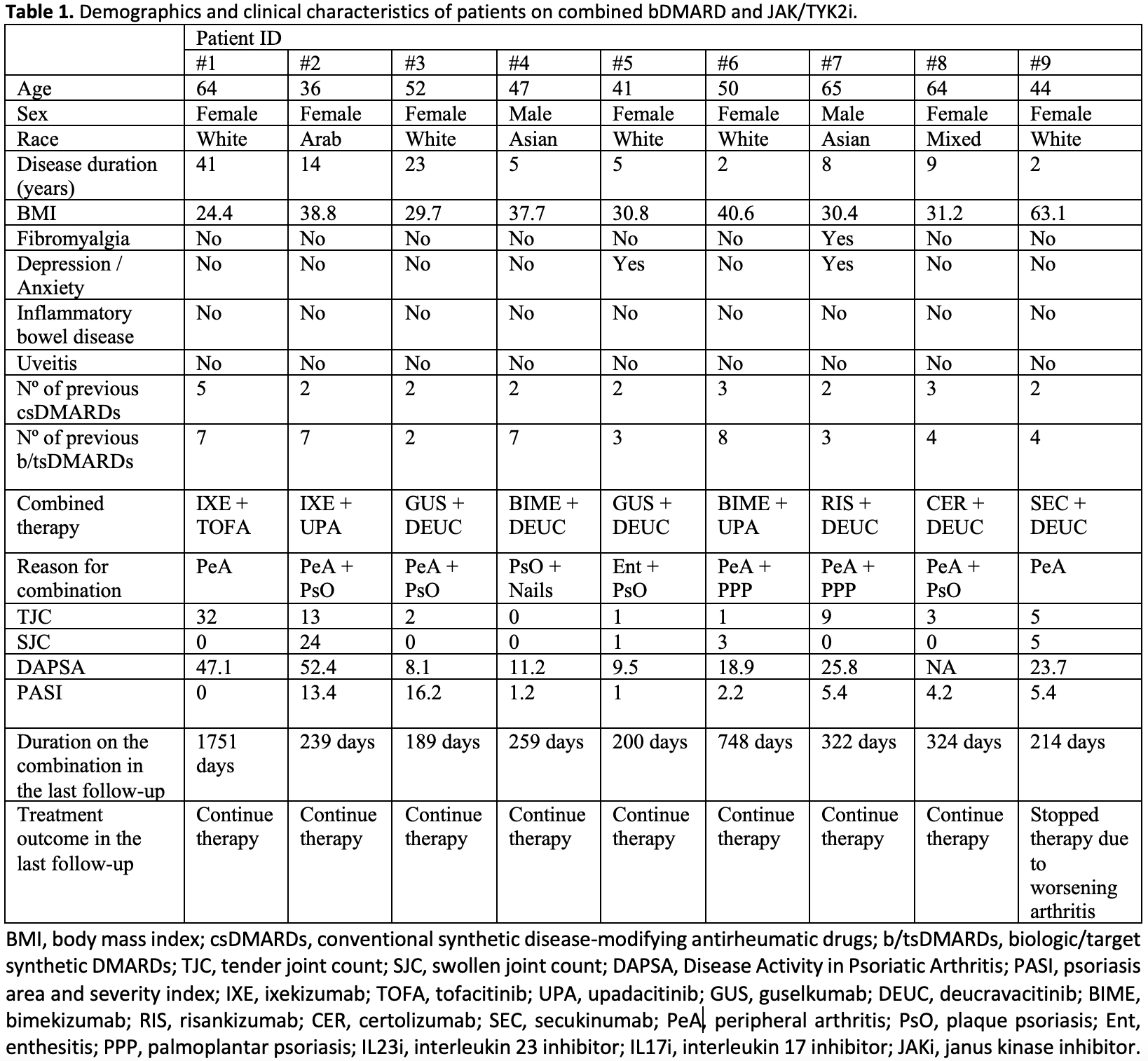
Methods: Case series within a prospective PsA cohort, identifying patients on combined b/tsDMARD therapy (two drugs from TNF inhibitors, JAKi, TYK2i, IL17i, IL23i, IL-12/23i). Apremilast (APR) and b/tsDMARD combinations were analyzed separately for safety.
Demographic and clinical data were collected prospectively and supplemented retrospectively via electronic records. Safety outcomes, including infections and non-infectious events, were reviewed.
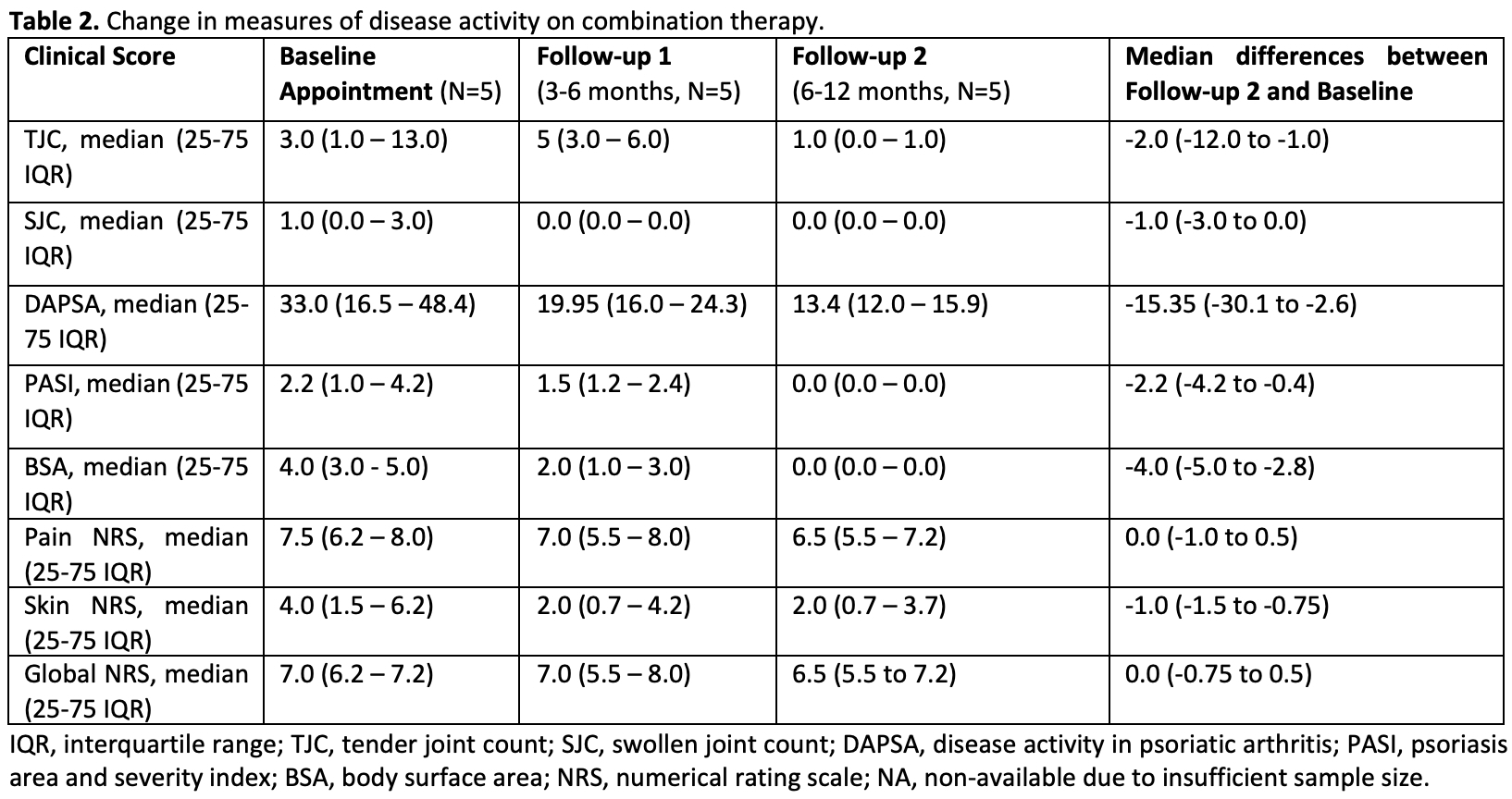
Treatment response was assessed through changes in disease activity scores from baseline to follow-ups at 3-6 and 6-12 months. Measurements included tender and swollen joint counts (TJC / SJC), Disease Activity in PsA (DAPSA), psoriasis area and severity index (PASI), body surface area (BSA), and patient-reported outcomes (numerical rating scale for pain, skin, and patient global).
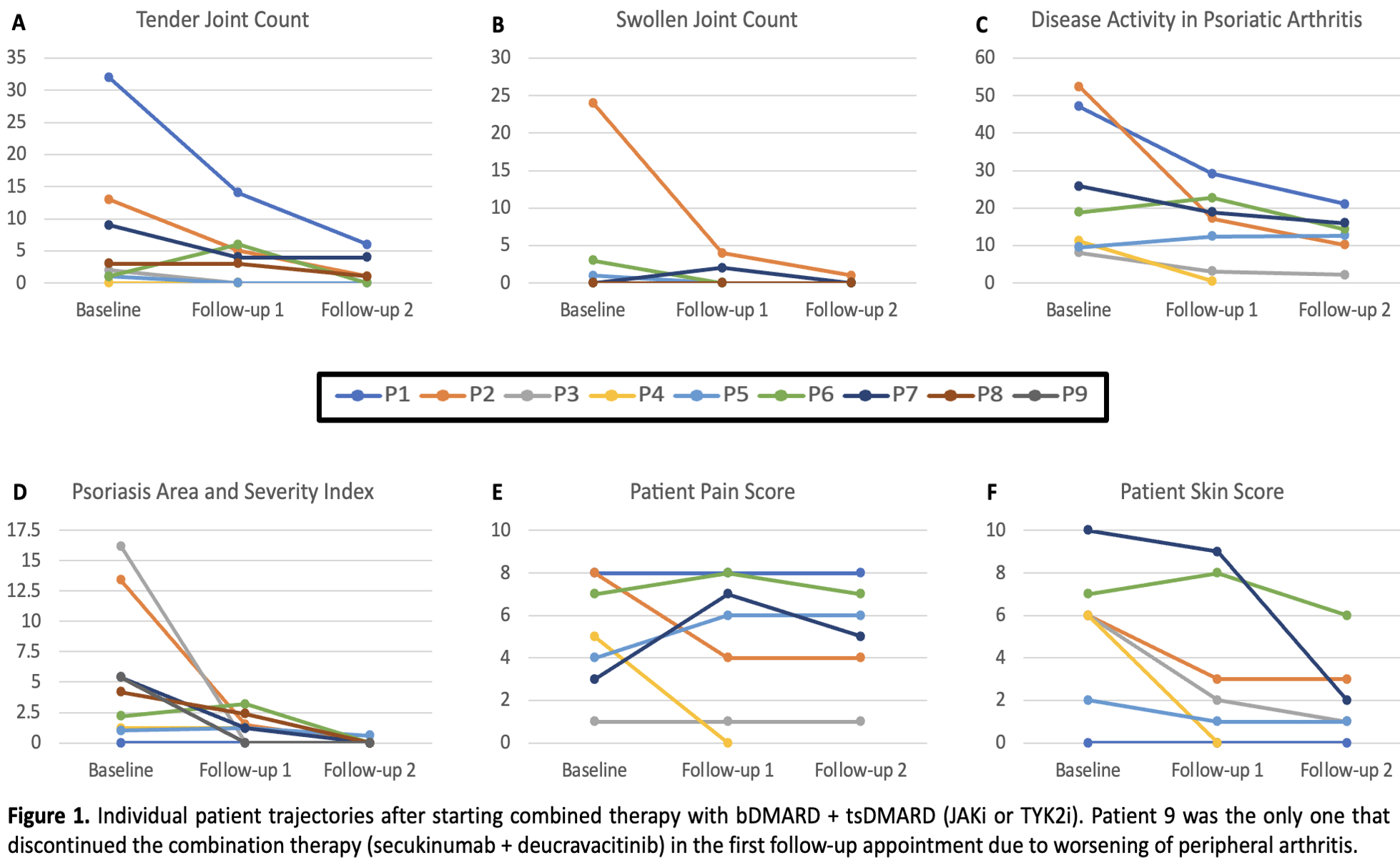
Results: We identified 9 patients that used combined JAK/TYK2i and bDMARD, detailed in Table 1. Most failed multiple csDMARDs (range 2-5) and b/tsDMARDs (range 2-8). The majority were obese (median BMI 31.2). The primary reason for combination therapy was active skin and musculoskeletal inflammation. Median duration on this combination therapy was 259 days. The IL17i + JAK/TYK2 was used in 5 patients (duration range of 214-1751 days), with one infectious stomatitis being observed. Four patients used IL23i + JAK/TYK2i combination (189-424 days), with one episode of folliculitis after addition of deucravacitinib. The TNFi + JAK/TYK2i combination was used 1 patient (324 days), with no side effects. Numerical improvement was seen across several measures of disease activity (Table 2 and Figure 2). Only 1 patient who was using ixekizumab plus deucravacitinib discontinued the therapy at the first follow-up due to worsening of the peripheral arthritis (the patient was previously using ixekizumab plus methotrexate).
We identified 15 patients using combinations of b/tsDMARDs with APR, with a median duration of 735 days. In the APR combination group, various therapies were used over different durations, with some patients having several different drugs combined with APR over time. IL12/23i or IL-23i + APR in 9 patients (360-1,890 days), IL17i + APR in 9 patients (90-2,790 days), TNFi + APR in 7 patients (180-2,340 days), and JAKi + APR in 1 patient (540 days). There were two episodes of diarrhea, with no infections.
Conclusion: This case series provides preliminary data demonstrating a favorable safety profile. Furthermore, short-term response was observed, with improvements in both musculoskeletal and skin domains. However, as this is an observational study with a short-term follow-up, there is a need for randomized clinical trials to further explore and validate these findings.
To cite this abstract in AMA style:
Lucas Ribeiro A, Carrizo Abarza V, Gladman D, Chandran V, Eder L. Combination of Biological and Targeted Synthetic Disease-Modifying Antirheumatic Drugs in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/combination-of-biological-and-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combination-of-biological-and-targeted-synthetic-disease-modifying-antirheumatic-drugs-in-psoriatic-arthritis/