Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Racial and ethnic minority groups face higher lupus prevalence and severity and remain inadequately represented in lupus clinical trials. Lupus Therapeutics, the clinical affiliate of the Lupus Research Alliance, oversees the Lupus Clinical Investigators Network (LuCIN), with 58 leading clinical research sites in North America. A network-wide annual survey is conducted to gain site perspectives on recruitment challenges and solutions for effective participant engagement in lupus clinical trials.
Methods: Survey questions were developed based upon current community considerations with input from multiple LuCIN/LRA contributors. Data from the 2024 LuCIN annual survey were collected between January 7, 2024 and February 18, 2024. The survey included several questions designed to assess views on barriers and facilitators to recruitment of underrepresented populations in lupus clinical trials, using descriptive statistics to determine trends in responses.
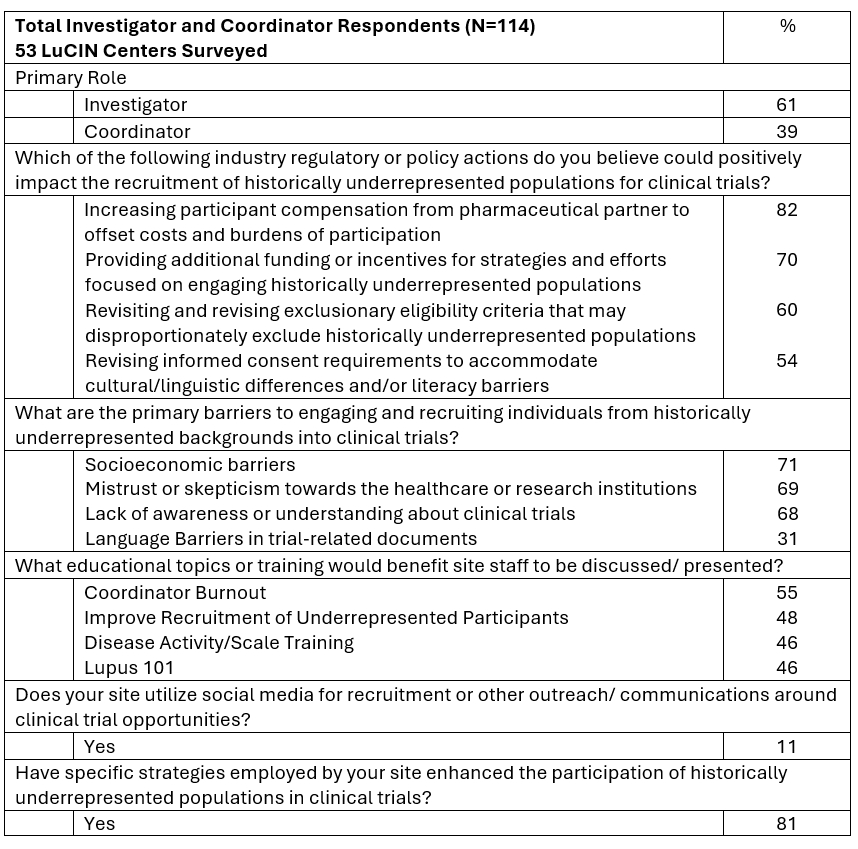
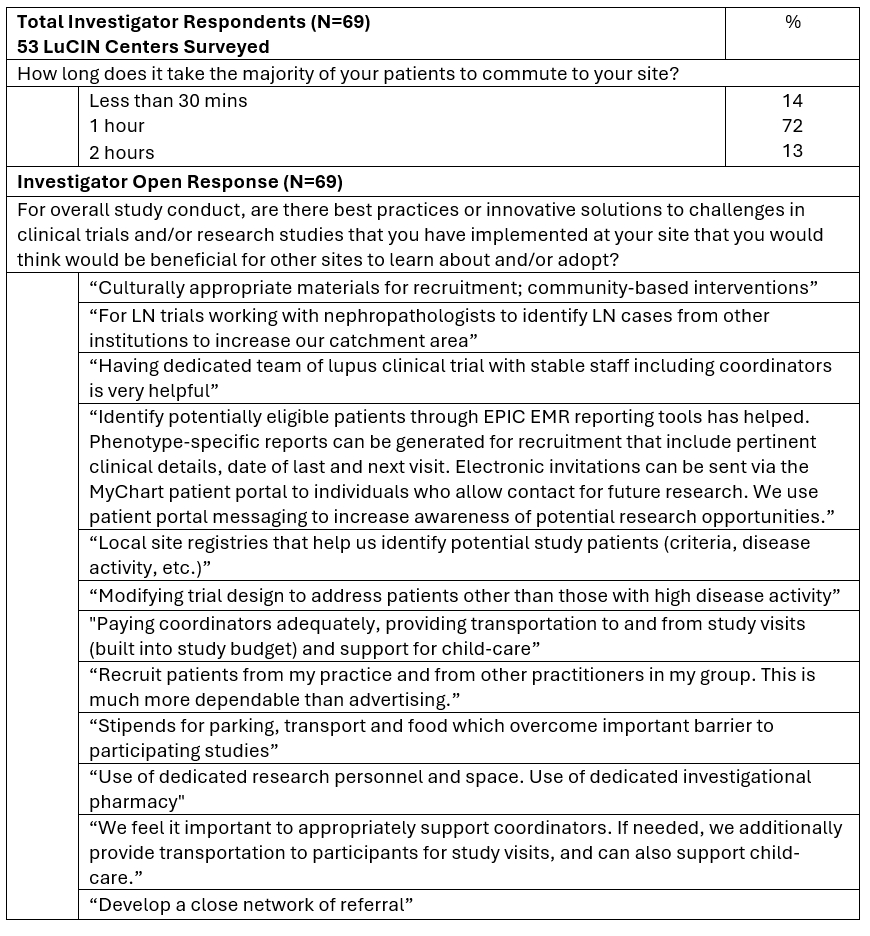
Results: 114 investigators and study coordinators highlighted strategies to improve clinical trial recruitment for underrepresented populations which include increasing patient compensation (82%), providing additional funding for engagement efforts (70%), and revising eligibility criteria (60%) Figure 1. Common perceived barriers to recruitment were socioeconomic (71%), institutional mistrust (69%) and lack of clinical trial awareness or understanding (68%) in addition to 86% of the investigators indicating long typical patient commute times of 1+ hours, bringing site accessibility concerns Figure 2. While most respondents (81%) reported successful strategies with potential to enhance participation of underrepresented populations in clinical trials, actual participation remains a challenge. Only 11% of respondents utilized social media for recruitment which marks an area of strategic opportunity. Educational training/topics that were most requested by sites should focus on coordinator burnout (55%) and recruitment training (48%), amongst others Figure 1. Investigators proposed innovative solutions to recruitment challenges which included maintaining sufficient staff, referral networks, increasing patient stipends and incentives, and enhancing community-based interventions Figure 2.
Conclusion: Perspectives provided by study teams across an omnipresent lupus clinical trials network in North America, serve to demonstrate the necessity for tailored, innovative strategies to increase engagement and enrollment of underrepresented populations. These findings highlight the need for increased patient compensation for study visits, funding for engagement opportunities and bolstering community-based initiatives to help address socioeconomic factors, institutional mistrust, and lack of clinical trial awareness and understanding. Future directions include an emphasis on enhancing support for research participants and sites, a focus on education and training to mitigate coordinator/staff burnout and implement social media strategies to improve recruitment. This work highlights tangible opportunities to promote equity in lupus clinical trials and favorably impact the future of lupus clinical research.
To cite this abstract in AMA style:
Jackson B, Dall'Era M, Sheikh S, Zhang X, Irons T, Finney C, Adjei T, Meriwether J, Donovan C, Menezes C, Bell S. Collaborative Solutions to Lupus Trial Challenges for Underrepresented Participant Recruitment & Engagement: Perspectives from the Lupus Clinical Investigators Network (LuCIN) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/collaborative-solutions-to-lupus-trial-challenges-for-underrepresented-participant-recruitment-engagement-perspectives-from-the-lupus-clinical-investigators-network-lucin/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/collaborative-solutions-to-lupus-trial-challenges-for-underrepresented-participant-recruitment-engagement-perspectives-from-the-lupus-clinical-investigators-network-lucin/