Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: Metabolic and Crystal Arthropathies – Basic and Clinical Science
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Treatment of acute CPP arthritis mainly relies on expert opinion, as there are no trials that assessed the efficacy of anti-inflammatory drugs, despite the high frequency of the disease. The objective of this study (COLCHICORT; clinicaltrial.gov: NCT03128905) was to test the equivalence between colchicine and prednisone in patients with acute CPPD arthritis.
Methods: This is a multicenter open-label randomized trial enrolling patients above 65 years of age and eGFR >30ml/min/1.73m2, presenting with an acute CPPD arthritis (duration of symptoms less than 36h), as defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on X-Rays or ultrasound.
Participants were randomized to receive either colchicine 1.5mg (1 mg and then 0.5 mg one hour later) at baseline and then 1mg on day 1 or oral prednisone 30mg at baseline and day 1. Patients were to receive systematically 1g of acetaminophen and 50mg tramadol TID during the first 24 hours. Patients were followed-up for 6 days. The primary outcome was change in pain (visual analogic scale (VAS); 0-100 mm)) at 24h. Equivalence between groups was set at less than 13 mm on the pain VAS at 24 h in the per-protocol analysis. Secondary outcomes were response to treatment defined by pain VAS improvement > 50% from baseline and/or a pain VAS < 40/100 at day 1 and 2. Adverse events (AEs) were assessed. A sample size of 56 participants per arm was calculated to demonstrate equivalence set at less than 13 mm on the pain VAS change at 24h with 80% power, two-sided type 1 error of 0.05, 10% loss to follow-up (1).
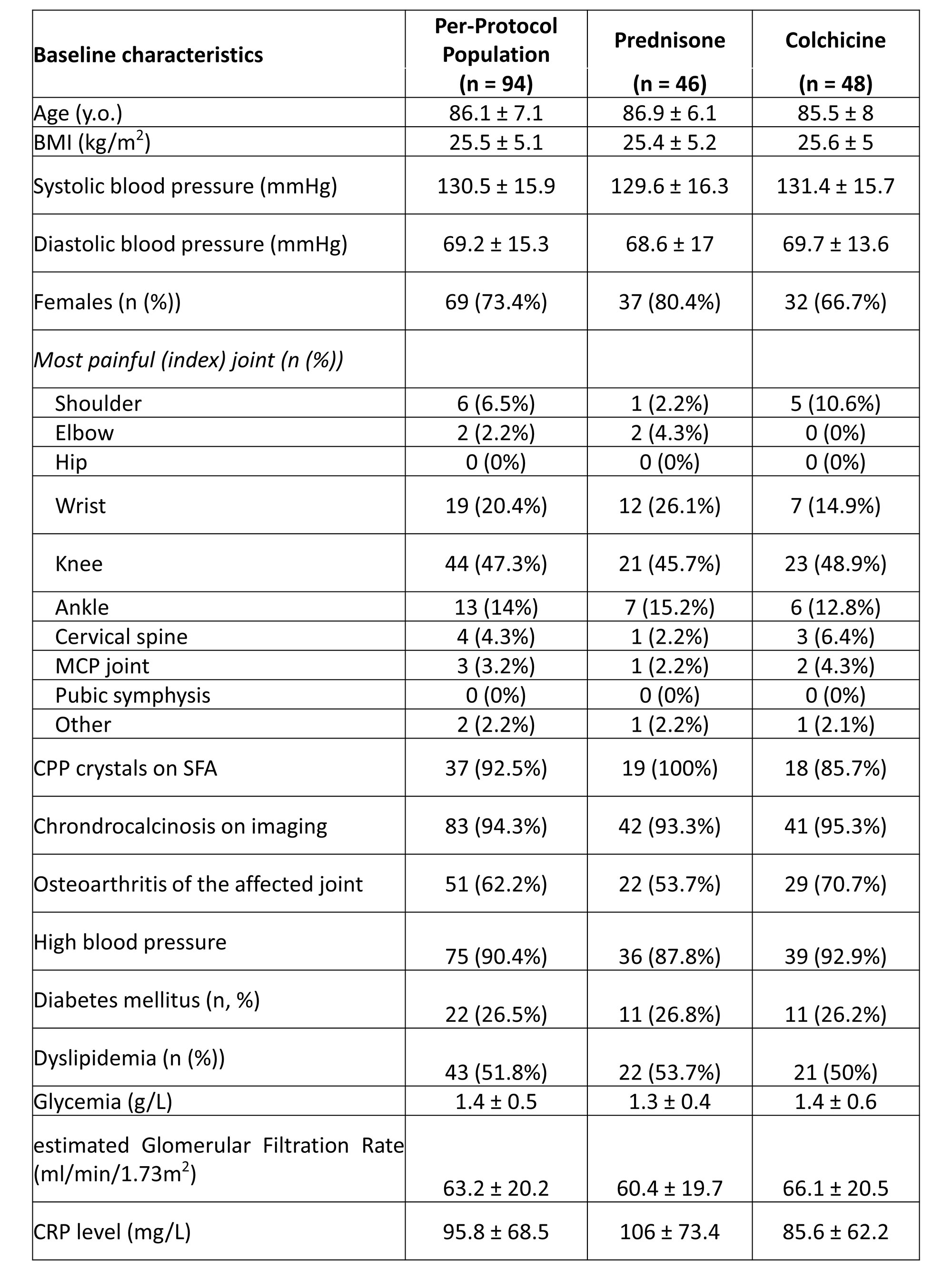
Results: A total of 112 hospitalized patients were randomized (mean age 86.1 ± 7.1 years; sex ratio F/M 73.4%)): n = 48 in the colchicine group and n = 46 in the prednisone group (per-protocol population (8 drop-outs, 10 major deviations)). Overall, 26.3% of participants had diabetes mellitus, and mean eGFR was 63.2 ± 20.2 ml/min/1.73m2. Acute CPPD arthritis was crystal-proven for n = 37 (39.4%), and affected mainly the knee (n = 44, 47.3%), the wrist (n = 19, 20.4%) and the ankle (n = 13, 14.0%)(Table 1).
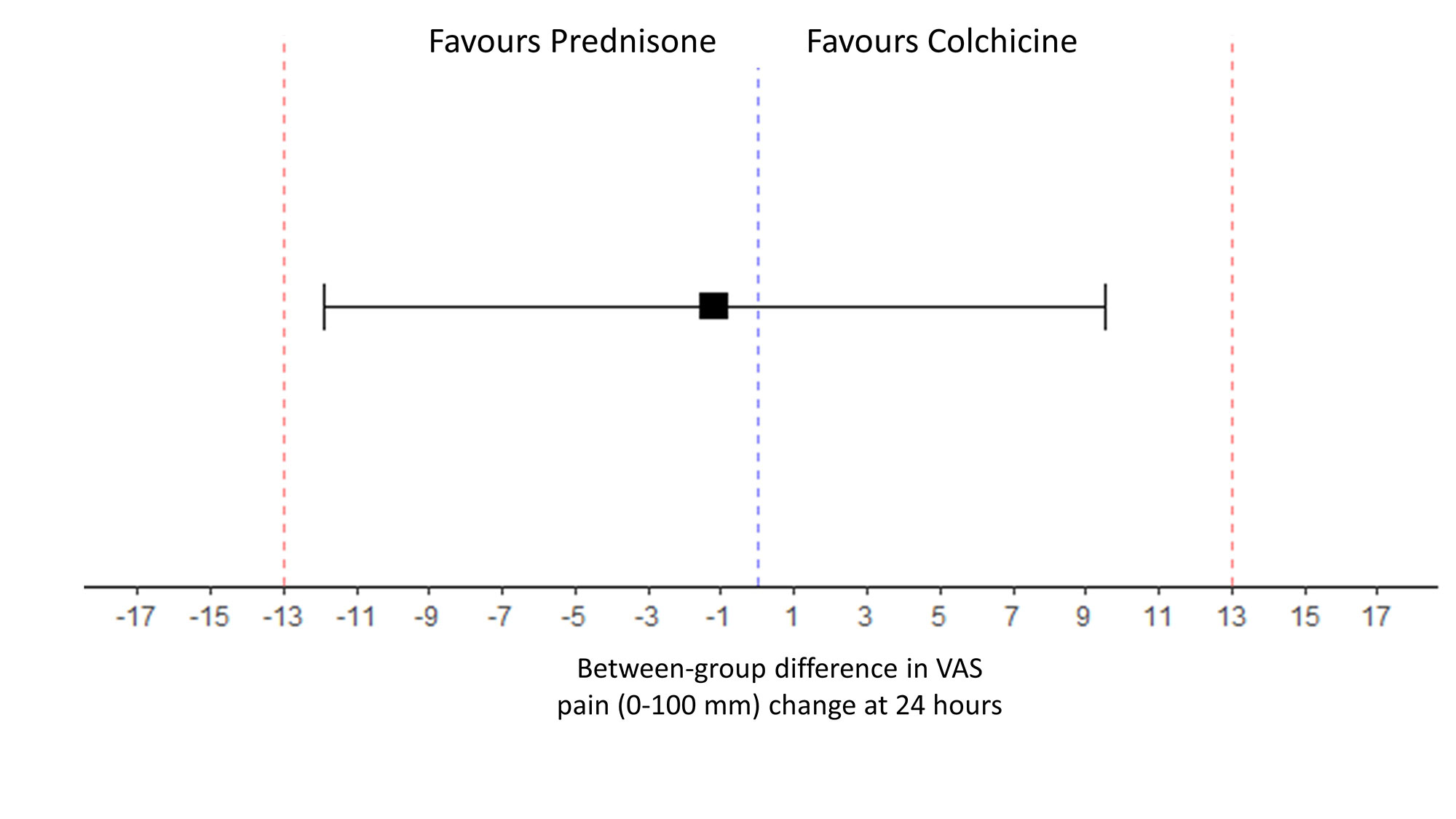
At 24 hours, change in pain VAS was -36.6 ±32.1 mm in the colchicine group and -37.7±19.4 in the prednisone group, respectively. The difference in pain change (VAS) at 24h between prednisone and colchicine was -1.17 mm [-11.88 ; 9.54] showing equivalence between the 2 drugs. (Figure 2).
Improvement in pain was similar between groups throughout the 6 days of follow-up (Figure 1). There were 64.6% of responders in the colchicine group and 66.7% in the prednisone group at 24h (p=1), and 63% and 72.7% in both groups (p=0.45) at 48h, respectively.
AEs were observed in n = 28 (50.9%) patients of the colchicine group and n = 20 (37%) in the prednisone group (p=0.21). The main AE in the colchicine group was diarrhea (n = 15, 26.3% vs n = 4, 7.3%) while it was onset of hypertension (n = 7, 12.7% vs n = 1, 1.8%), hyperglycemia (n = 3, 5.5% vs n = 0, 0%), and insomnia (n = 3, 5.5% vs n = 0, 0%) in the prednisone group, all resolving.
Conclusion: This randomized trial showed efficacy equivalence of low dose colchicine and oral prednisone in acute CPP arthritis.
Reference
1. Todd KH, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27:485-9.
To cite this abstract in AMA style:
Pascart T, Robinet P, Ottaviani S, Leroy R, Segaud N, Pacaud A, Luraschi H, Rabin T, Grandjean A, Deplanque X, Maciejasz P, Visade F, Mackowiak A, Baclet N, Lefebvre A, Budzik J, Bardin T, Richette P, Norberciak L, Ducoulombier V, Houvenagel E. Colchicine or Prednisone for the Treatment of Acute Calcium Pyrophosphate Deposition Arthritis: A Multicenter Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/colchicine-or-prednisone-for-the-treatment-of-acute-calcium-pyrophosphate-deposition-arthritis-a-multicenter-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/colchicine-or-prednisone-for-the-treatment-of-acute-calcium-pyrophosphate-deposition-arthritis-a-multicenter-randomized-controlled-trial/