Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Systemic Sclerosis and Related Disorders – Clinical I: Trials and Therapeutics
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Enrichment strategies from clinical trials for progressive systemic sclerosis-associated interstitial lung disease (SSc-ILD) have been partly successful but have not been tested in a real life cohort. The objective of this study was to analyse the feasibility, representativeness and efficacy of enrichment strategies in SSc-ILD patients from the EUSTAR cohort.
Methods: We applied the (partly modified) inclusion criteria of major recent SSc-ILD trials (focuSSced, SLS II and SENSCIS) in SSc-ILD patients and assessed progressive ILD, defined as absolute change in forced vital capacity (FVC) and as significant progression (FVC decline >10%) over time. Data were compared to patients not fulfilling any inclusion criteria.
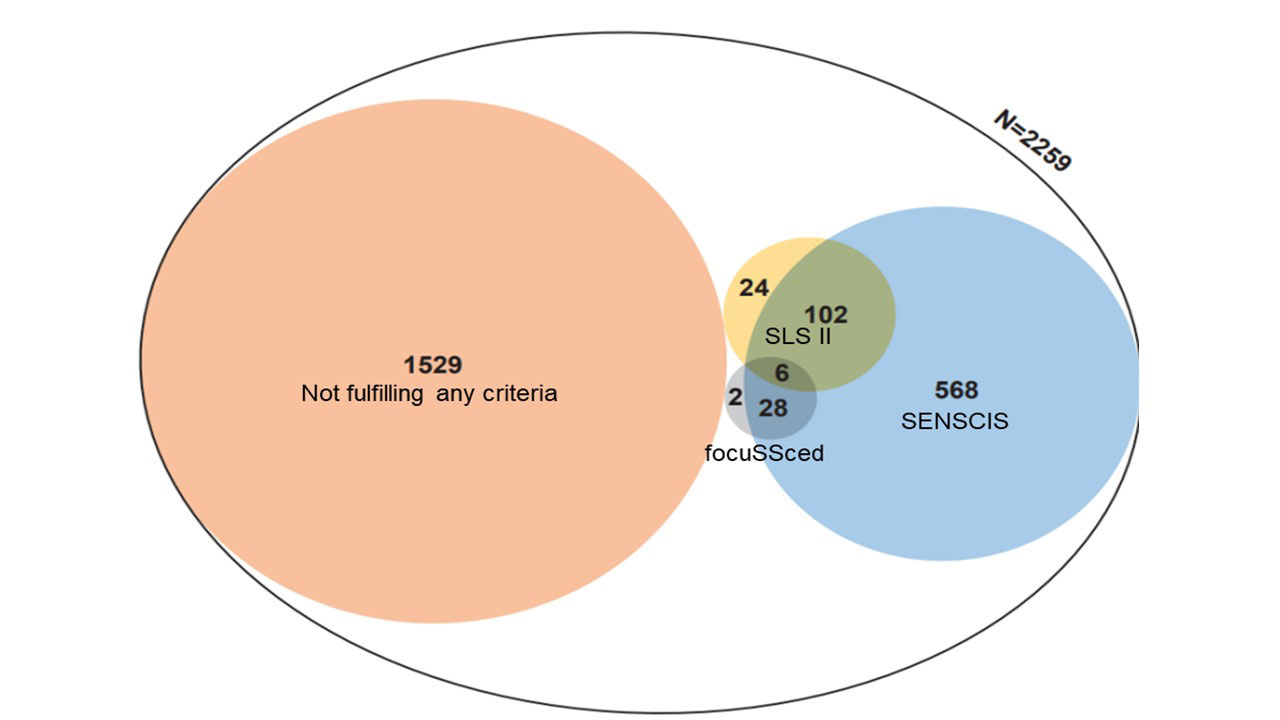
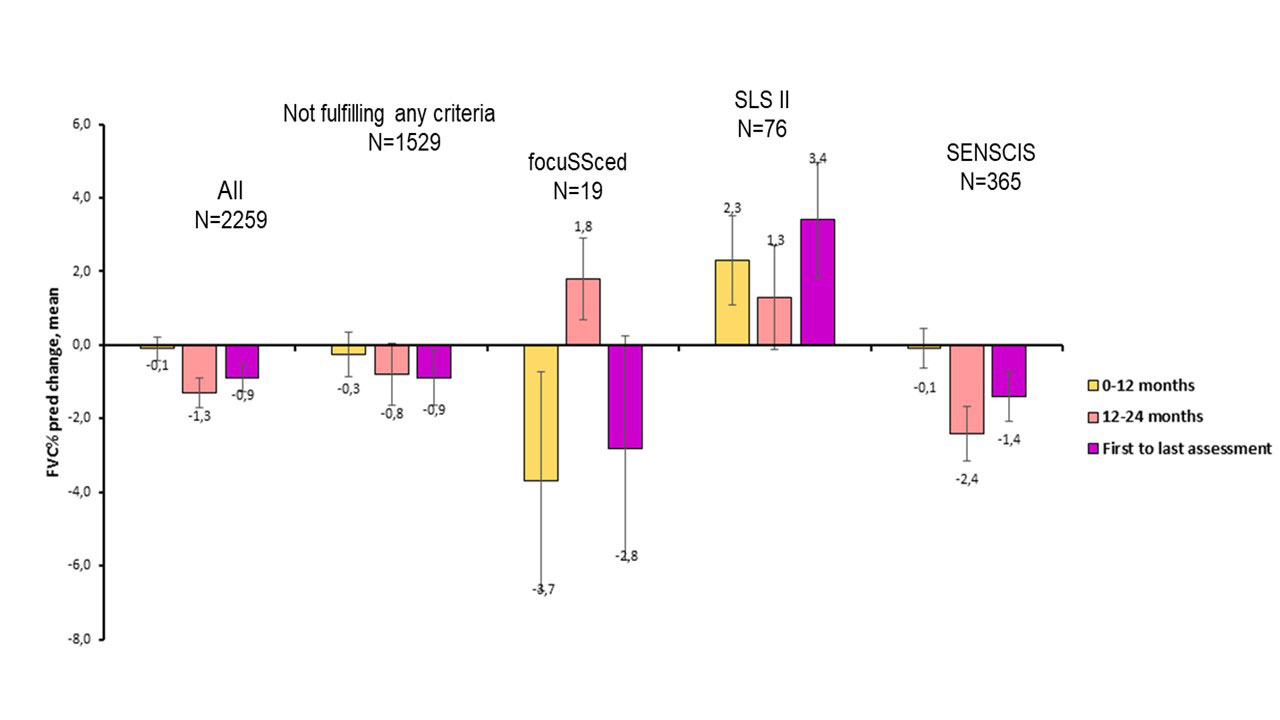
Results: In total, 2258 SSc-ILD patients were included, with 31.2% meeting SENSCIS, 5.8% SLS II, 1.6% focuSSced criteria and 1529 (67.7%) not meeting any criteria, highlighting reduced feasibility of recruitment of SSc-ILD patients into clinical trials (Figure 1). We also showed a selection of patient populations with changed representativeness (Table). In the first 12+/-3 months, a slow FVC% decline of –0.1% was seen in the total, unselected cohort and in patients fulfilling SENSCIS criteria. Patients fulfilling criteria from focuSSced showed a strong FVC decline of –3.7%. Notably, patients enriched for SLS II criteria showed FVC improvement of +2.3% (Figure 2). Similarly, compared to the total unselected cohort, the number of significant progressive events was numerically higher in patients fulfilling focuSSced criteria, the same for SENSCIS criteria and even slightly lower for patients fulfilling the SLS2 criteria
In the second 12 months period, SENSCIS enriched patients had a further absolute FVC% decline as described for the total cohort. In contrast, patients fulfilling the focuSSced and SLS II inclusion criteria showed numerical improvement of lung function in the second period (Figure 2). There were no significant associations of enrichment criteria and ILD progression in the second period.
Over the mean observation period of 2.3 years, patients not fulfilling any inclusion criteria showed the same FVC decline of –0.9 (12.1) as observed for the total cohort (–0.9% (12.6)). There were numerical differences in FVC changes in the enriched patient cohorts, varying from –2.8% FVC decline in patients fulfilling the focuSSced criteria to +3.4% FVC improvement with SLS II criteria.
Conclusion: Application of enrichment criteria from previous clinical trials showed enrichment for progression with variable success but led to selected patient populations reducing feasibility of recruitment and representativeness of the SSc-ILD patients. These findings are important for future clinical trial design and interpretation of the results of published trials.
To cite this abstract in AMA style:
Hoffmann-Vold A, Brunborg C, Airò P, Ananyeva L, Czirják L, Guiducci S, Hachulla E, LI M, Mihai C, Riemekasten G, Sfikakis P, Valentini G, Kowal-Bielecka O, Allanore Y, Distler O. Cohort Enrichment Strategies for Progressive Interstitial Lung Disease in Systemic Sclerosis from EUSTAR [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/cohort-enrichment-strategies-for-progressive-interstitial-lung-disease-in-systemic-sclerosis-from-eustar/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cohort-enrichment-strategies-for-progressive-interstitial-lung-disease-in-systemic-sclerosis-from-eustar/