Session Information
Date: Saturday, November 16, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
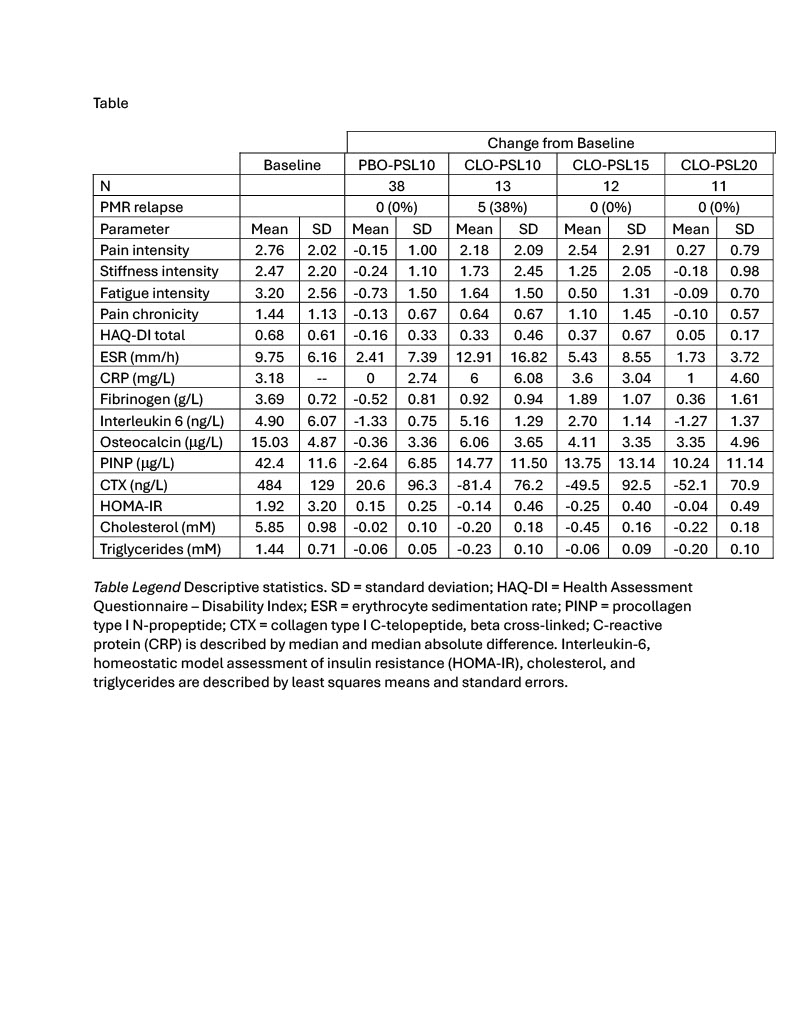
Background/Purpose: 11β-hydroxysteroid dehydrogenase type 1 (HSD-1) differentially regulates intracellular glucocorticoid levels in the immune system and glucocorticoid toxicity target organs. Clofutriben is a potent HSD-1 inhibitor. We sought to observe whether clofutriben can both maintain prednisolone efficacy and reduce prednisolone toxicity in patients with polymyalgia rheumatica (PMR).
Methods: Patients with PMR and who received prednisolone 10 mg/day continued treatment for 4 weeks without dose reduction. They additionally received clofutriben 6 mg/day or matching placebo (PBO-PSL10) for 2 weeks each. In sequential cohorts, during clofutriben treatment the prednisolone dose was 10 (CLO-PSL10), 15 (CLO-PSL15), or 20 mg/day (CLO-PSL20). Relapse was defined when the investigator further increased the prednisolone dose to manage a participant’s PMR symptoms. Participants completed daily numeric rating scales for pain, stiffness, and fatigue intensity, and pain chronicity, and Health Assessment Questionnaire-Disability Index during trial visits. Inflammatory, bone, and lipid biomarkers were measured at Baseline, Week 2, and Week 4. Insulin resistance (HOMA-IR) was calculated as fasting glucose
insulin. Trial interpretation is based on descriptive statistics. Hepatic HSD-1 inhibition was monitored via urinary metabolites: (tetrahydrocortisol+allotetrahydrocortisol)/tetrahydrocortisone.
Results: Clofutriben achieved 94.7±4.6% hepatic HSD-1 inhibition. Relapse incidence and descriptive statistics for other parameters are presented (Table). PMR therapeutic control was reduced with CLO-PSL10 and CLO-PSL15, but not CLO-PSL20, compared to PBO-PSL10. Substantial improvements were observed on bone, lipid, and glycemic control parameters related to prednisolone toxicity with each clofutriben-containing regimen.
Conclusion: In patients with PMR the combination of clofutriben and prednisolone 20 mg, compared to prednisolone 10 mg alone, showed an improved benefit-risk profile comprised of similar efficacy and less evidence of prednisolone toxicity.
To cite this abstract in AMA style:
DR. BUTTGEREIT F, Everding A, Andreica I, Kellner H, Schuch F, Marmon T, Czerwiec F, Desai K, Katz D. Clofutriben to Improve the Benefit-Risk Profile of Prednisolone in Patients with Polymyalgia Rheumatica [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clofutriben-to-improve-the-benefit-risk-profile-of-prednisolone-in-patients-with-polymyalgia-rheumatica/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clofutriben-to-improve-the-benefit-risk-profile-of-prednisolone-in-patients-with-polymyalgia-rheumatica/