Session Information
Date: Saturday, November 16, 2024
Title: B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: CD19-directed CAR T cell therapy has been reported to induce profound B cell depletion and deep clinical responses, including drug-free remission, in patients with refractory autoimmune diseases, most notably systemic lupus erythematosus (SLE), rheumatoid arthritis, and scleroderma. However, the approach poses significant logistical challenges and safety risks. TCEs offer an alternative approach to T cell redirecting therapy with similarly effective B cell depletion but improved safety and off-the-shelf convenience. CLN-978 is a fusion protein comprised of tandemly arranged CD19- and CD3-specific single-chain fragment variable domains (scFvs) and a single-domain albumin binder to prolong serum half-life. CLN-978 has high affinity for CD19 (KD = 73.8 pM) and moderate affinity for CD3 (KD =7.18 nM), and shows an approximately 10-fold lower potency for cytokine induction than for lysis of CD19+ target cells (Meetze et al 2023). CLN-978 is administered via subcutaneous (SC) injection. Additional preclinical, translational, and clinical studies were conducted to further characterize the activity of CLN-978.
Methods: Peripheral blood mononuclear cells (PBMC) from healthy and SLE donors were cultured in the presence of CLN-978 for 48 hours. Cynomolgus monkeys were administered 4 weekly SC doses of CLN-978. In a phase 1, first-in-human study of CLN-978 (NCT05879744), three patients with B cell non-Hodgkin lymphoma (B-NHL) were treated with 30 mg SC weekly of CLN-978.
Results: In vitro, CLN-978 induced relatively similar B cell killing, T cell activation and cytokine production in PBMCs from healthy donors and SLE patients.
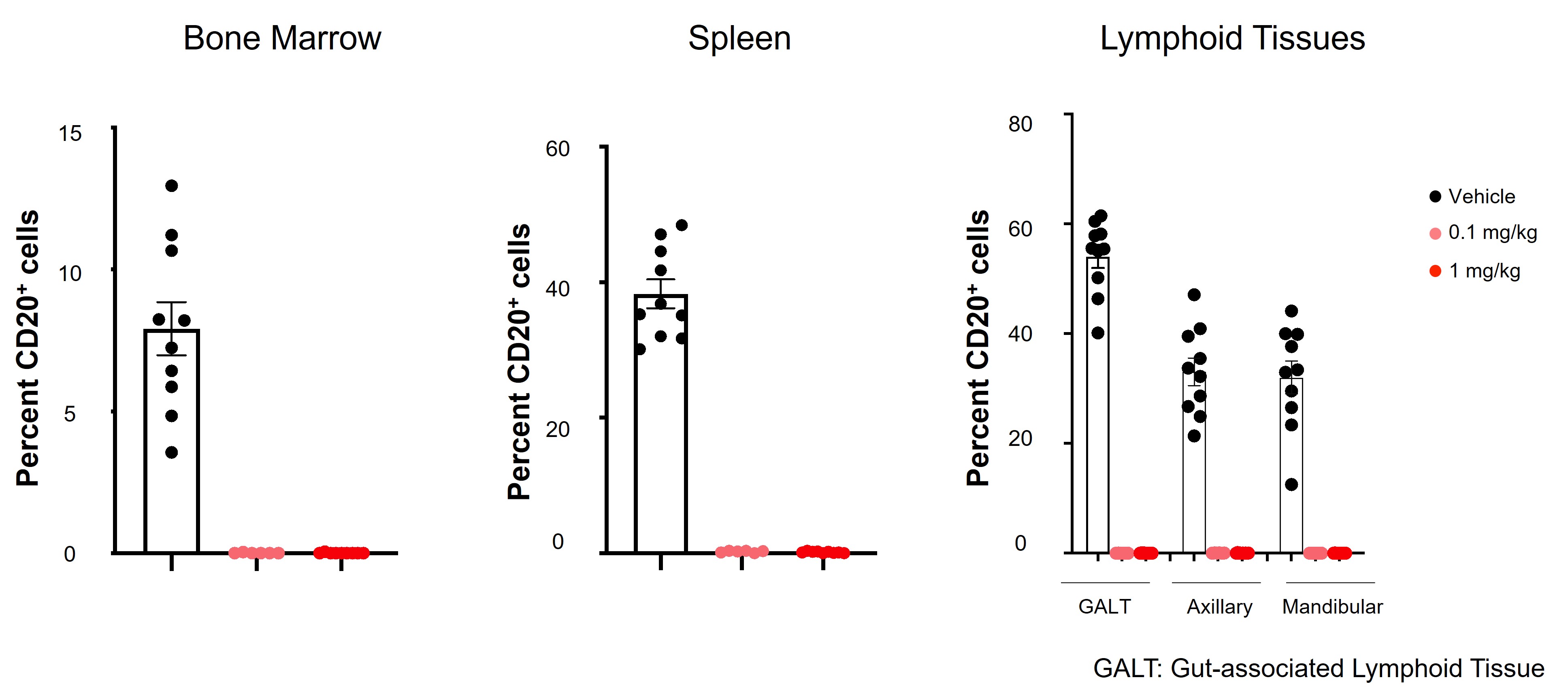
In cynomolgus monkeys treated with CLN-978, deep B cell depletion was observed in peripheral blood as well as in tissues including bone marrow, spleen and lymphoid tissues (Figure 1). CLN-978 was well tolerated in monkeys following administration of multiple weekly SC doses.
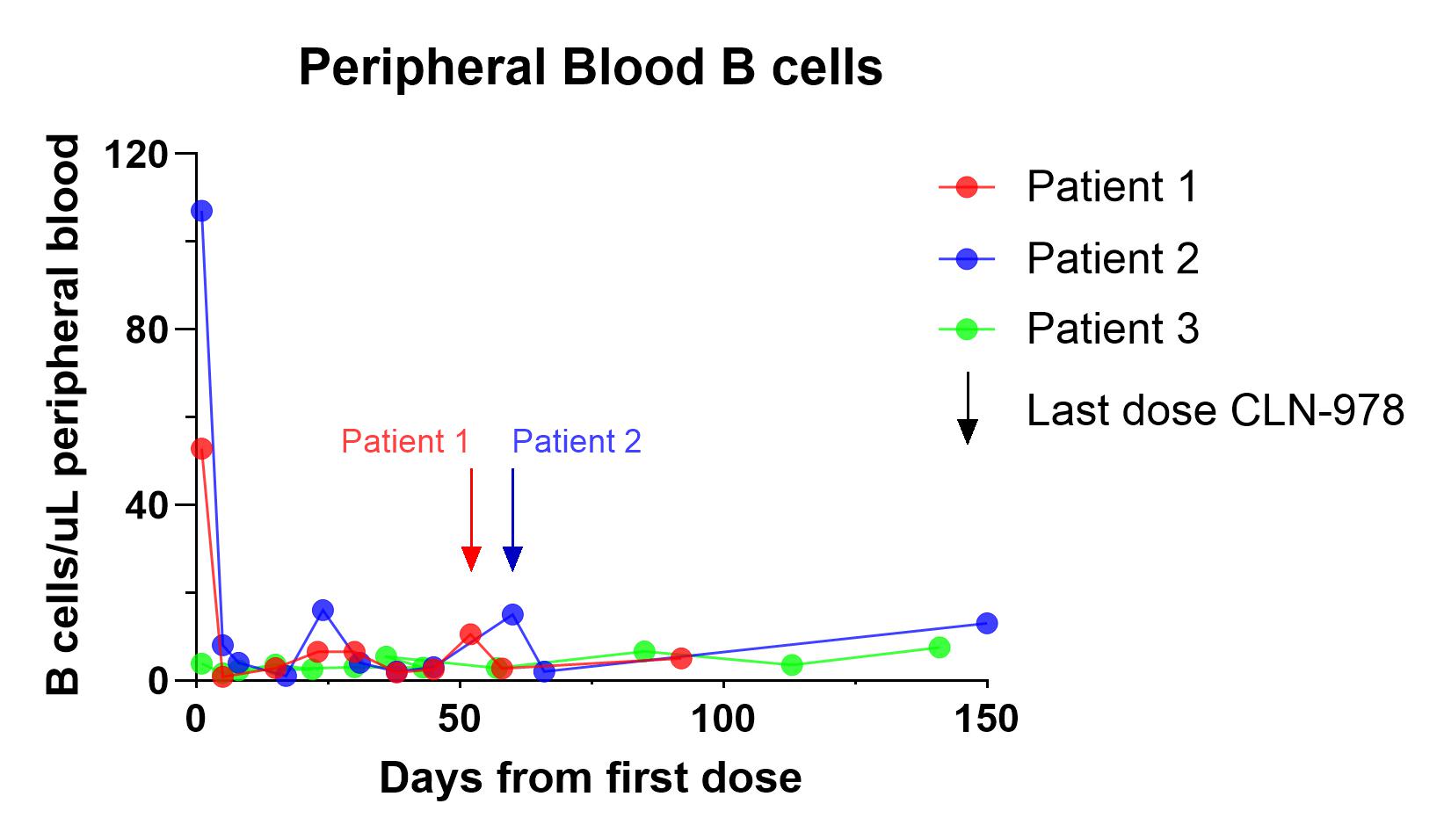
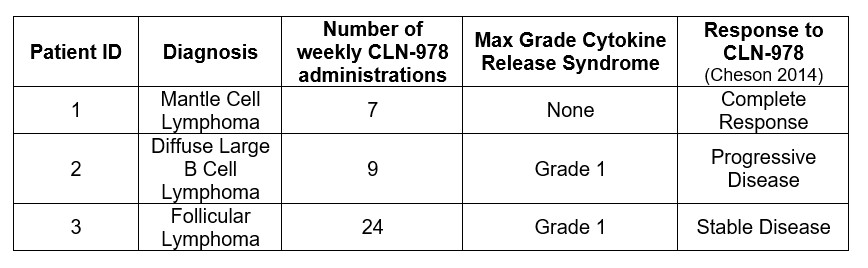
In a phase 1, first-in-human study of CLN-978, three patients with B-NHL received weekly SC injections of 30 µg CLN-978. Within 96 hours following the first dose, peripheral B cells were depleted by 93% and 98% in the 2 patients with detectable baseline levels of B cells (Figure 2). Two patients with bulky lymphoma experienced Grade 1 cytokine release syndrome (CRS) following only the first administration of CLN-978. A complete metabolic (FDG-PET) response was observed in one patient who did not develop CRS (Table 1).
A phase 1b study is planned with CLN-978 in SLE patients.
Conclusion: CLN-978 is a highly potent, SC-administered, CD19-directed TCE with both preclinical and clinical data providing a strong rationale for broad development in autoimmune diseases which benefit from B cell depletion.
To cite this abstract in AMA style:
Quigley M, Michaelson J, Jones J, Awan F, Shouse G, Zhang Y, Shearer T, Inumerable J, Shapiro I, Baeuerle P, Wax S. CLN-978, a CD19-directed T Cell Engager (TCE), Leads to Rapid and Deep B Cell Depletion and Has Broad Potential for Development in Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cln-978-a-cd19-directed-t-cell-engager-tce-leads-to-rapid-and-deep-b-cell-depletion-and-has-broad-potential-for-development-in-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cln-978-a-cd19-directed-t-cell-engager-tce-leads-to-rapid-and-deep-b-cell-depletion-and-has-broad-potential-for-development-in-autoimmune-diseases/