Session Information
Date: Friday, November 6, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Multimorbidity
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Lymphoproliferative disorders (LPDs) in patients with rheumatoid arthritis (RA) are one of potentially life-threatening complications. However, the diagnosis of LPDs is complicated by the diversity in clinical, laboratory, and pathological manifestations. As the data on LPDs in RA patients are limited to small-scale studies, we aimed to clarify their clinicopathological characteristics in a large multicenter study.
Methods: We retrospectively reviewed the clinical, laboratory, and pathological data of adult patients with RA who were newly diagnosed as having LPDs between January 2000 and March 2017 in eight tertiary hospitals in Japan. In addition to patients with biopsy-proven LPDs, we included patients clinically diagnosed as having LPD (clinical LPD) without biopsy because of prompt regression of clinical manifestations after withdrawal of immunosuppressive therapy.
Results: Of the 232 patients with LPDs, the median age was 67 years (IQR 60–73 years), and 77.1% were female. At the time of LPD diagnosis, 94.4% of the patients received methotrexate. The development of LPDs was observed from early to long-standing RA, irrespective of disease duration (Table 1). Approximately 60% of patients were in remission or low disease activity status based on the DAS28-CRP with three variables. B symptoms, lymphadenopathy, and extranodal involvement were present in 30.6%, 77.3%, and 50.7% of the patients, respectively (Table 2). Of the 230 patients with available data, 55 (23.9%), 54 (23.5%), 47 (20.4%), and 74 (32.2%) were in the Ann Arbor stages I, II, III, and IV, respectively. Of the 116 patients with extranodal involvement, the most frequently involved sites were the lungs (36 [30.2%]), oropharyngeal mucosa (19 [16.4%]), bone marrow (14 [12.1%]), skin (13 [11.2%]), and gastrointestinal tract (12 [10.3%]). Lymphocytopenia (≤1,000/mm3) was present in 44.7% of the patients, elevated lactate dehydrogenase (≥230 U/L) in 53.4%, elevated C-reactive protein (≥0.30 mg/dL) in 75.2%, and elevated soluble interleukin-2 receptor (≥500 U/mL) in 82.9%. Biopsy was performed in 195 patients (84.1%). The most common LPD pathological subtype was diffuse large B-cell lymphoma (79 [40.5%]), followed by classic Hodgkin lymphoma (21 [10.8%]), Epstein-Barr virus-positive mucocutaneous ulcer (EBVMCU) (15 [7.7%]), reactive lymphoid hyperplasia (12 [6.2%]), unclassifiable B-cell lymphoma (11 [5.6%]), and follicular lymphoma (10 [5.1%]). The clinical and laboratory characteristics were diverse across the pathological subtypes (Table 3).
Conclusion: LPDs occurred mainly in patients aged 50 years or older who were receiving methotrexate. Although the clinical manifestations of LPDs vary depending on the pathological subtypes, lymphadenopathy, extranodal mass, and mucocutaneous ulcer should be recognized as possible early signs of LPDs in combination with laboratory abnormalities in patients with RA.
 Demographic and clinical characteristics of RA patients with LPDs
Demographic and clinical characteristics of RA patients with LPDs
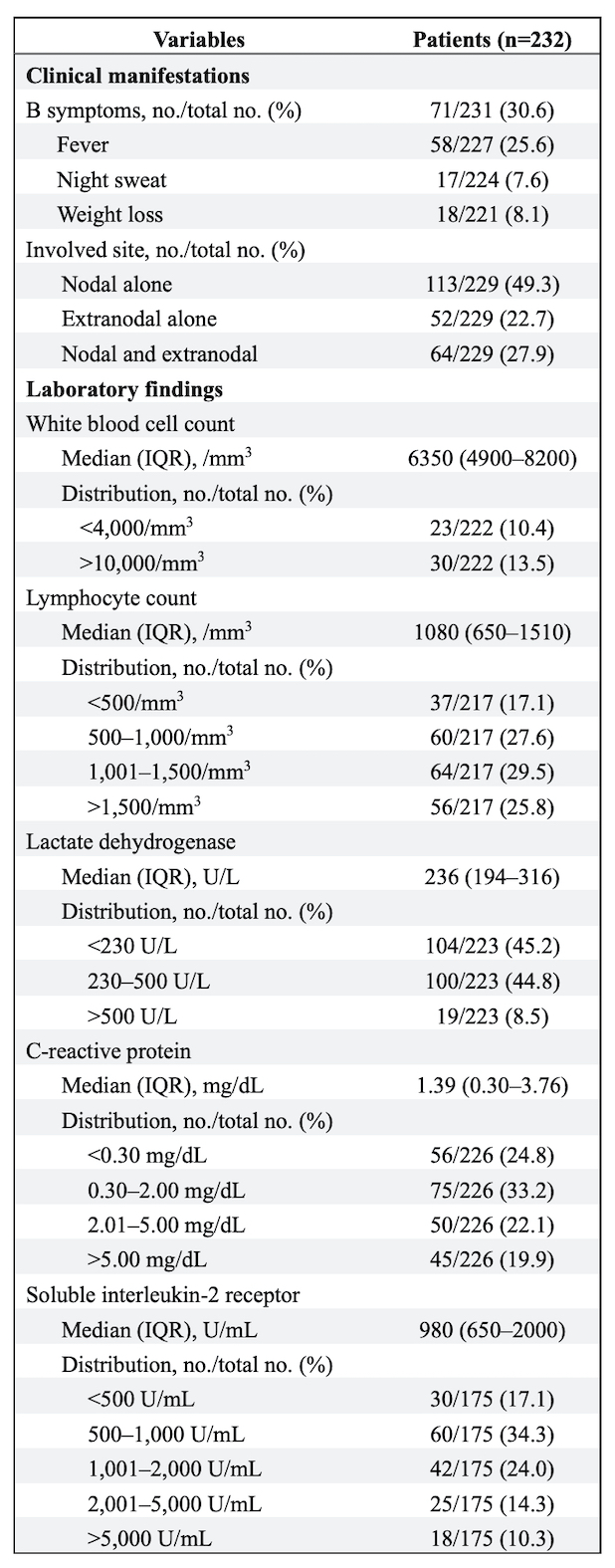 Clinical manifestations of LPDs and laboratory findings in patients with RA
Clinical manifestations of LPDs and laboratory findings in patients with RA
 Clinical characteristics, laboratory findings, and EBV-encoded small RNA (EBER) positivity in each pathological subtype of LPDs in patients with RA
Clinical characteristics, laboratory findings, and EBV-encoded small RNA (EBER) positivity in each pathological subtype of LPDs in patients with RA
To cite this abstract in AMA style:
Takada H, Kaneko Y, Nakano K, Tanaka M, Fujii T, Saito K, Sugimoto N, Sasaki S, Saito S, Saito R, Kuramoto N, Suzuki Y, Harigai M. Clinicopathological Characteristics of Lymphoproliferative Disorders in 232 Patients with Rheumatoid Arthritis in Japan [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/clinicopathological-characteristics-of-lymphoproliferative-disorders-in-232-patients-with-rheumatoid-arthritis-in-japan/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinicopathological-characteristics-of-lymphoproliferative-disorders-in-232-patients-with-rheumatoid-arthritis-in-japan/
