Session Information
Date: Monday, November 11, 2019
Title: Pediatric Rheumatology – ePoster II: SLE, Juvenile Dermatomyositis, & Scleroderma
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Corticosteroids (CS) are the mainstay of childhood-onset systemic lupus erythematosus (cSLE) and proliferative lupus nephritis (LN) therapy. However, there are no widely accepted CS dosing regimens for the treatment of LN. As a first step to establish CS dosing standards for cSLE and proliferative LN, we aimed to identify clinical variables influencing CS dosing with focus on oral prednisone dosing in children with newly diagnosed proliferative LN.
Methods: Consensus formation techniques were used. Patient profiles (PP) were generated using data from the medical records of children with proliferative LN at Cincinnati Children’s Hospital Medical Center (CCHMC). Using the extracted data, an online survey was sent to rheumatologists (PP raters) at CCHMC and the University of Cincinnati Medical Center (UCMC) to adjudicate on renal and extra-renal clinical disease courses and estimate prednisone dosing for a given PP. Pearson correlation coefficients were computed to identify clinical variables that correlate with the estimated prednisone doses by PP raters. Mixed model analysis was performed to determine multivariable predictors of the estimated prednisone dose.
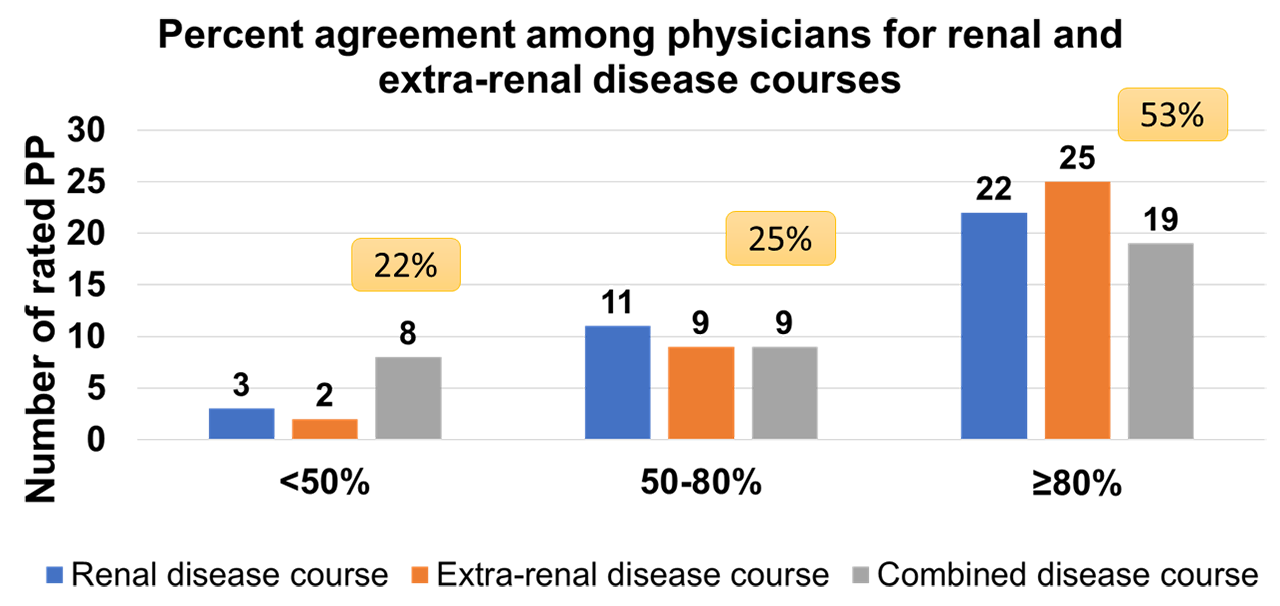
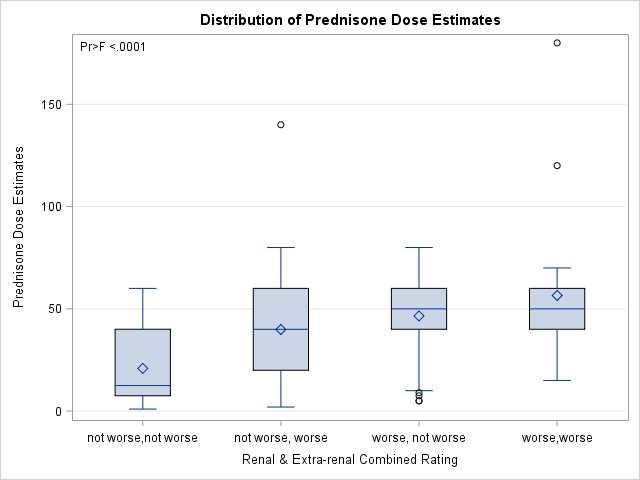
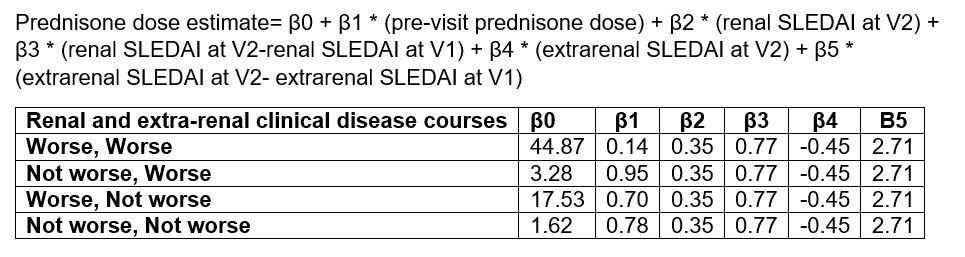
Results: Overall, the survey completion rate was 87% with a total of 291 completed PP. Each PP provides information about 2 subsequent visits (V1 and V2). PP survey responses showed a high level of agreement among raters for renal and extra-renal clinical disease courses when categorized into worsening vs non-worsening disease (Figure 1). The estimated prednisone dose by PP raters is found to be most strongly correlated with the pre-visit prednisone dose (r=0.70), and C3 level at V2 (r= -0.56). Proteinuria and SLEDAI-2K scores at V2 show moderate correlations with the estimated prednisone dose (r=0.49, and r=0.48 respectively). Using one-way ANOVA, the highest mean prednisone dose estimates are noted for worsening of the combined renal and extra-renal disease courses (57 ± 34 mg) and the lowest when the combined disease courses are not worse (21 ± 18 mg) (Figure 2). The pre-visit prednisone dose and the PP ratings of renal, extra-renal and combined renal and extra-renal clinical disease courses are found to be the best predictors of prednisone dose estimates using mixed model analysis (Table 1).
Conclusion: Prednisone dosing regimens in children with newly diagnosed proliferative LN depend on physicians’ opinions of renal, extra-renal and the combined clinical disease courses as well as the pre-visit prednisone dose. The identification of clinical variables that correlate with and predict prednisone dosing regimens is key to the future development of CS treatment algorithms in children with proliferative LN.
To cite this abstract in AMA style:
Chalhoub N, Qiu T, Deng J, Merritt A, Huang B, Brunner H. Clinical Variables Influencing Prednisone Dosing Towards the Development of Corticosteroid Treatment Algorithms in Pediatric Proliferative Lupus Nephritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinical-variables-influencing-prednisone-dosing-towards-the-development-of-corticosteroid-treatment-algorithms-in-pediatric-proliferative-lupus-nephritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-variables-influencing-prednisone-dosing-towards-the-development-of-corticosteroid-treatment-algorithms-in-pediatric-proliferative-lupus-nephritis/