Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Evidence suggests that timely and effective management can improve long-term outcomes in patients (pts) with psoriatic arthritis (PsA); however factors influencing treatment management decisions are not well understood. The objective of this study is to evaluate the association between the clinical specialty setting and time from inflammatory musculoskeletal symptom onset to PsA diagnosis and to different management steps in pts with a diagnosis of PsA.
Methods: LOOP is a large cross-sectional, multi-center observational study currently being conducted in 19 countries across Western and Eastern Europe, Latin America, and Asia. Adult pts (≥18 years) with a suspected or an established diagnosis of PsA who are routinely visiting a rheumatologist (rheum), dermatologist (derm) or non-rheum/non-derm site are eligible to participate in this study. Each enrolled patient is being assessed by both rheum and derm in the study. The present interim analysis included pts enrolled by 23 May 2017, which corresponded to approximately 50% of the total protocol specified sample size.
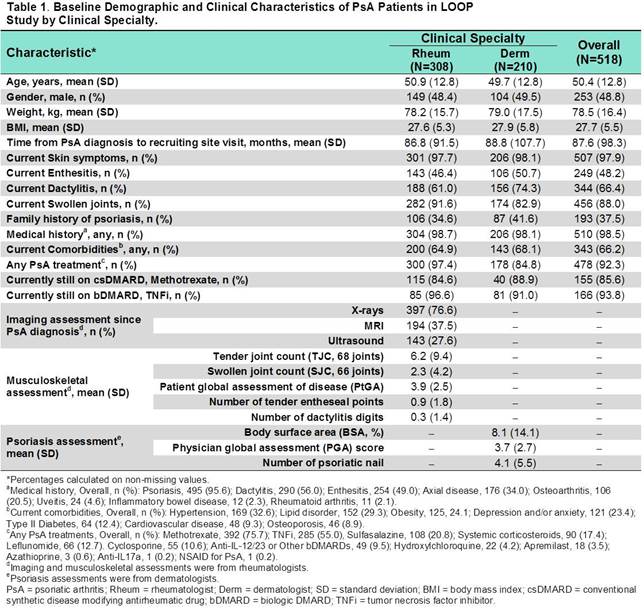
Results: Of the 602 pts enrolled by database cut-off date, 518 pts with a confirmed diagnosis of PsA were included in this interim analysis. A majority of pts were recruited by rheums (308, 59.5%), followed by derms (210, 40.5%) and physiatrists (19, 3.7%). PsA was first diagnosed by a rheum in 372 (71.8%) pts and by a derm in 100 pts (19.3%). Baseline demographics and disease characteristics were mostly comparable between PsA pts in rheum and derm settings (Table 1). The timing of different disease management steps by clinical specialty is reported in Table 2. The mean time from symptom onset to PsA diagnosis was 23 months (mo) in the rheum setting and 2 mo longer for derms. The mean time from PsA diagnosis to first conventional synthetic DMARD (csDMARD) and first biologic DMARD (bDMARD) for rheums were 9 and 53 mo, respectively. Compared with Rheums, Derms required additional 16 and 14 mo to prescribe first csDMARD (P = 0.015 vs Rheum) and first bDMARD, respectively. The mean time from first csDMARD to first bDMARD was 46 mo for rheum; while it was 6 months shorter for derm.
Conclusion: Although the duration from musculoskeletal symptom onset to PsA diagnosis was similar between rheum and derm setting, there were differences in the timing of introduction of different DMARD classes. Notably, mean time to first csDMARD was significantly and mean time to first bDMARD numerically shorter in rheum setting. The data lend further support to the need for rheum-derm collaborative approach to optimize management of pts with PsA.
To cite this abstract in AMA style:
Boehncke WH, Horváth R, Dalkiliç E, Lima SAL, Okada M, Hojnik M, Ganz F, Lubrano E. Clinical Specialty Setting As a Determinant for Disease Management in Patients with Psoriatic Arthritis: An Interim Analysis of the Cross-Sectional Observational Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/clinical-specialty-setting-as-a-determinant-for-disease-management-in-patients-with-psoriatic-arthritis-an-interim-analysis-of-the-cross-sectional-observational-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-specialty-setting-as-a-determinant-for-disease-management-in-patients-with-psoriatic-arthritis-an-interim-analysis-of-the-cross-sectional-observational-study/