Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The management of patients with early rheumatoid arthritis (RA), defined by the American College of Rheumatology (ACR) guidelines as patients who have had RA disease or symptoms for < 6 months, can improve prognosis and long-term clinical outcomes and reduce joint damage. In the Middle East, available evidence suggests that the management of patients with RA may be suboptimal, indicating a need for guidelines supporting the early use of biologic DMARDs.
Methods: This observational, retrospective study assessed real-world patient data entered in the National Center of Rheumatology database in Iraq from 2012 till 2018. Patients included in the study after they met ACR/EULAR 2010 criteria for Rheumatoid Arthritis with 1 year of follow up after starting their first etanercept therapy. Patients are excluded from the study if previously or currently treated with other biological therapies. Patients were categorized into two separate groups:
- Patients were stratified based on the disease duration before initiation of treatment with etanercept (≤1 year, >1 to ≤4 years, >4 to ≤10 years, >10 to ≤20 years, and >20 years) (group A).
- Patients were stratified based on the mean duration of RA: early and delayed treatment initiation were defined as having a RA diagnosis below or above mean of RA duration, before initiation of treatment with etanercept (group B).
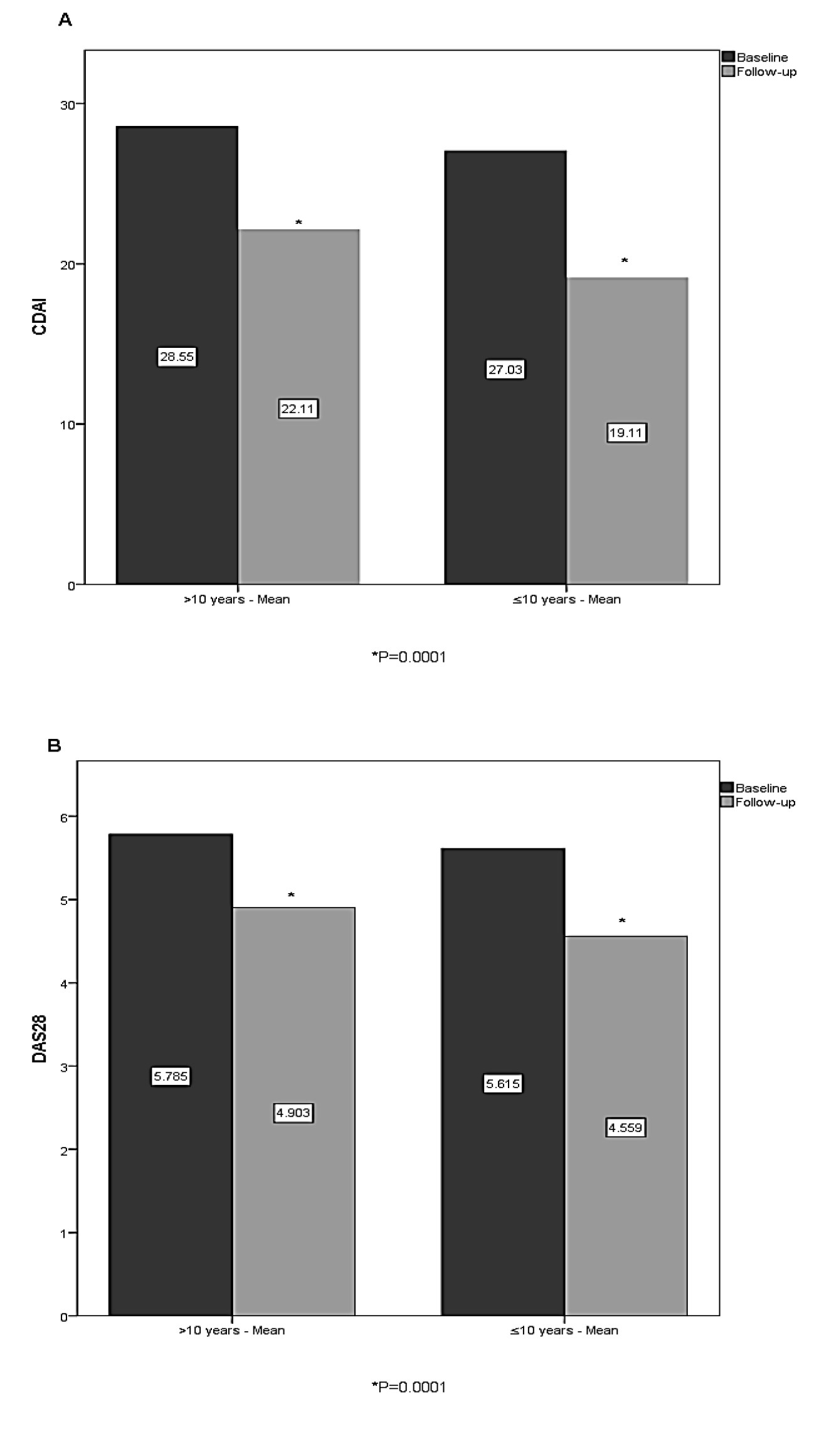
Results: A total of 979 Iraqi patients with RA (of whom 813 [83%] were female) were included in the analysis, with a mean RA duration at baseline of 10 years and mean Clinical Disease Activity Index (CDAI) and Disease Activity Score 28 (DAS28) scores of 27.65 ± 11.63 and 5.68 ± 1.2, respectively (Table 1). Based on the stratified group (A), Patients who had RA symptoms for ≤1 year before etanercept initiation had the greatest CDAI change from baseline (10.9- point) and it was significantly lower in comparison to another stratified group (P=0.01). The change from baseline for >1 to ≤4 years, >4 to ≤10 years, >10 to ≤20 years, and >20 years group were (7.32-, 7.69-, 6.41-, and 6.62- point decrease respectively) [Figure 1A]. The mean change in DAS28 score was 1.1- point decrease for patients who had RA symptoms for ≤1 year and >1 to ≤4 years before etanercept initiation, while other patients ( >4 to ≤10 years, >10 to ≤20 years, and >20 years groups) showed mean change of (1-, 0.83-, and 0.9- point decrease respectively) [Figure 1B]. In group B, at the last follow-up visit, patients with early etanercept initiation (≤10 years) had a higher decrease in CDAI and DAS28 scores from baseline compared with patients with delayed initiation ( >10 years) (CDAI 7.9-point and CDAI 6.4-point decrease, respectively) [Figure 2A], (DAS28 1.05-point and DAS28 0.88-point decrease, respectively) [Figure 2B].
Conclusion: Patients who received earlier etanercept treatment appear to have a better clinical outcome compared with patients with delayed treatment.
 Table 1. Summary of baseline characteristics
Table 1. Summary of baseline characteristics
 Figure 1. Changes in CDAI (A) and DAS28 (B) scores from baseline in patients stratified by RA duration before initiation of treatment with etanercept
Figure 1. Changes in CDAI (A) and DAS28 (B) scores from baseline in patients stratified by RA duration before initiation of treatment with etanercept
To cite this abstract in AMA style:
Abdulateef N, Gorial F, Humadi Y, Yasiry D, Al Derwibee F, Sunna N, AlJabban A. Clinical Outcomes of Earlier versus Delayed Treatment of Iraqi Patients with Rheumatoid Arthritis with Etanercept [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/clinical-outcomes-of-earlier-versus-delayed-treatment-of-iraqi-patients-with-rheumatoid-arthritis-with-etanercept/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-of-earlier-versus-delayed-treatment-of-iraqi-patients-with-rheumatoid-arthritis-with-etanercept/