Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: The spread of COVID-19 began in December 2019 and quickly escalated into a global pandemic resulting in millions of deaths. Many factors are associated with disease severity including older age, obesity, lung disease and cardiovascular disease. However, data regarding severity in patients with autoimmune inflammatory rheumatic diseases (AIIRD) is limited. Despite the positive impact of the COVID-19 vaccine, patients may be reluctant to get vaccinated. In this study, we aim to establish the impact of vaccination in patients with AIIRD in our clinics, including adverse events, frequency of disease flare and/or changes in the DMARD after vaccination and insight to obstacles preventing vaccine administration. The study is divided into two phases: the initial COVID-19 vaccine and the booster vaccine.
Methods: This is a cross-sectional study of patients with AIIRD aged 18 or older who were seen at Tampa General and University of South Florida Clinics. Phase 1 was conducted from June 2021 to November 2021, while Phase 2 was done from January 2022 to May 2022. Patients received a questionnaire asking vaccination status, disease flares, adverse events, modifications in medication regimen and concerns regarding vaccination if they opted not to receive it.
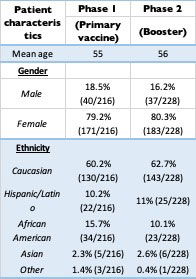
Results: A total of 444 patients participated in the study. There were 216 in the first phase (18.5% male, 79.2% female). The second phase included 228 patients (16.2% male, 80.3% female). Most of the patients were Caucasian in both groups (approximately 60%), followed by African American (less than 15%). 83% in both groups received the first course of COVID-19 vaccine, while only 16% declined. Among second phase participants who received the vaccine, 18% of patients did not receive the booster. The most common diagnosis was RA (28%, 126/444) followed by PsA (11.5%, 51/444) and SLE (11%, 49/444). Approximately 50% (214/444) in both groups reported not modifying their DMARD at the time of vaccine or booster.
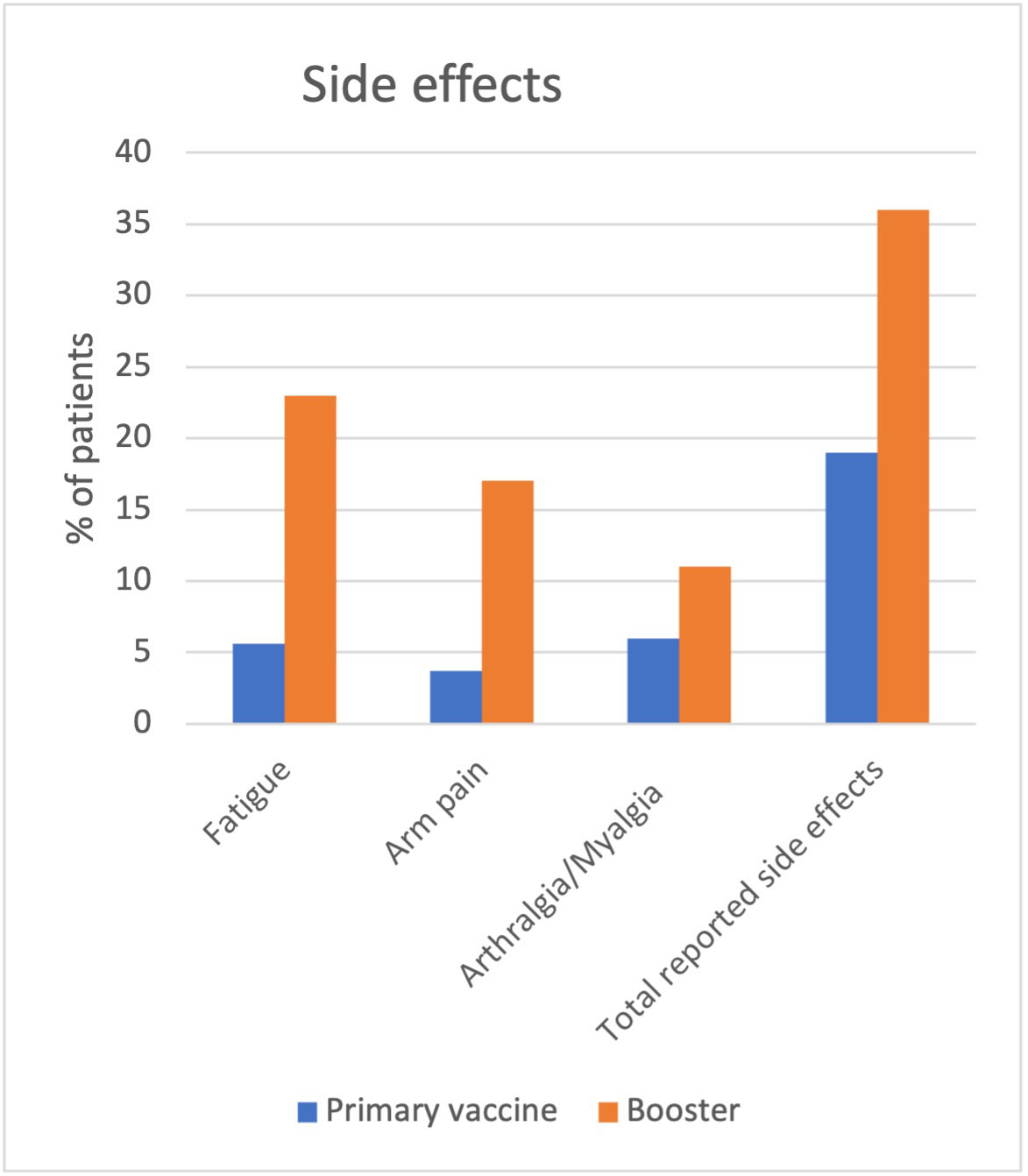
In the first phase, 19% reported side effects (42/216) versus 36% after receiving the booster (83/228), which was statistically significant (p = 0.00). The most frequent side effects included fatigue (5.6% vs 23%), arm pain (3.7% vs 17%), and arthralgias and myalgias (6% vs 11%). Out of all the 70% patients in both phases who answered, only 7% reported a flare of their rheumatic disease, both after the initial COVID-19 vaccination and the booster.
Conclusion: Our study shows a statistically significant difference between side effects of initial COVID-19 vaccination versus the booster, with the latter group having significantly higher rates. Interestingly, there was no significant difference between rheumatic disease flares between both groups. Additional information about the impact of COVID-19 vaccination versus the booster in patients with AIIRD is still needed, especially in terms of potential adverse events.
To cite this abstract in AMA style:
Holguin Balbuena S, Radisic A, Goodman S, Gor S, Wood B, Shahin A, Samuel K, Mhaskar R, Carter J. Clinical Outcomes of COVID-19 Vaccination and Booster in Patients with Autoimmune Inflammatory Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/clinical-outcomes-of-covid-19-vaccination-and-booster-in-patients-with-autoimmune-inflammatory-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-of-covid-19-vaccination-and-booster-in-patients-with-autoimmune-inflammatory-rheumatic-diseases/