Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders II: Clinical
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Relapsing polychondritis (RP) is a rare systemic inflammatory disease without standard treatment guidelines. This study aims to investigate the clinical characteristics, current treatment approaches, and association between clinical manifestations and immunomodulatory medication use in patients with RP.
Methods: This study included adult patients with physician-diagnosed RP enrolled in a multicenter prospective observational cohort. Data collected at baseline included demographics, clinical manifestations of RP ever experienced, disease damage indices, and immunomodulatory medications ever received. History of immunomodulatory treatment was categorized into three groups: I) no treatment or treatment only with glucocorticoids (GCs); II) treatment with non-biologic immunosuppressive (IS) drugs [other than Janus kinase inhibitors (JAKis)] with/ without GCs; III) treatment with JAKis and/or biologic IS with/without GCs/non-biologic IS. Chi-square test and logistic regression were used to determine the association between categorical and continuous independent variables.
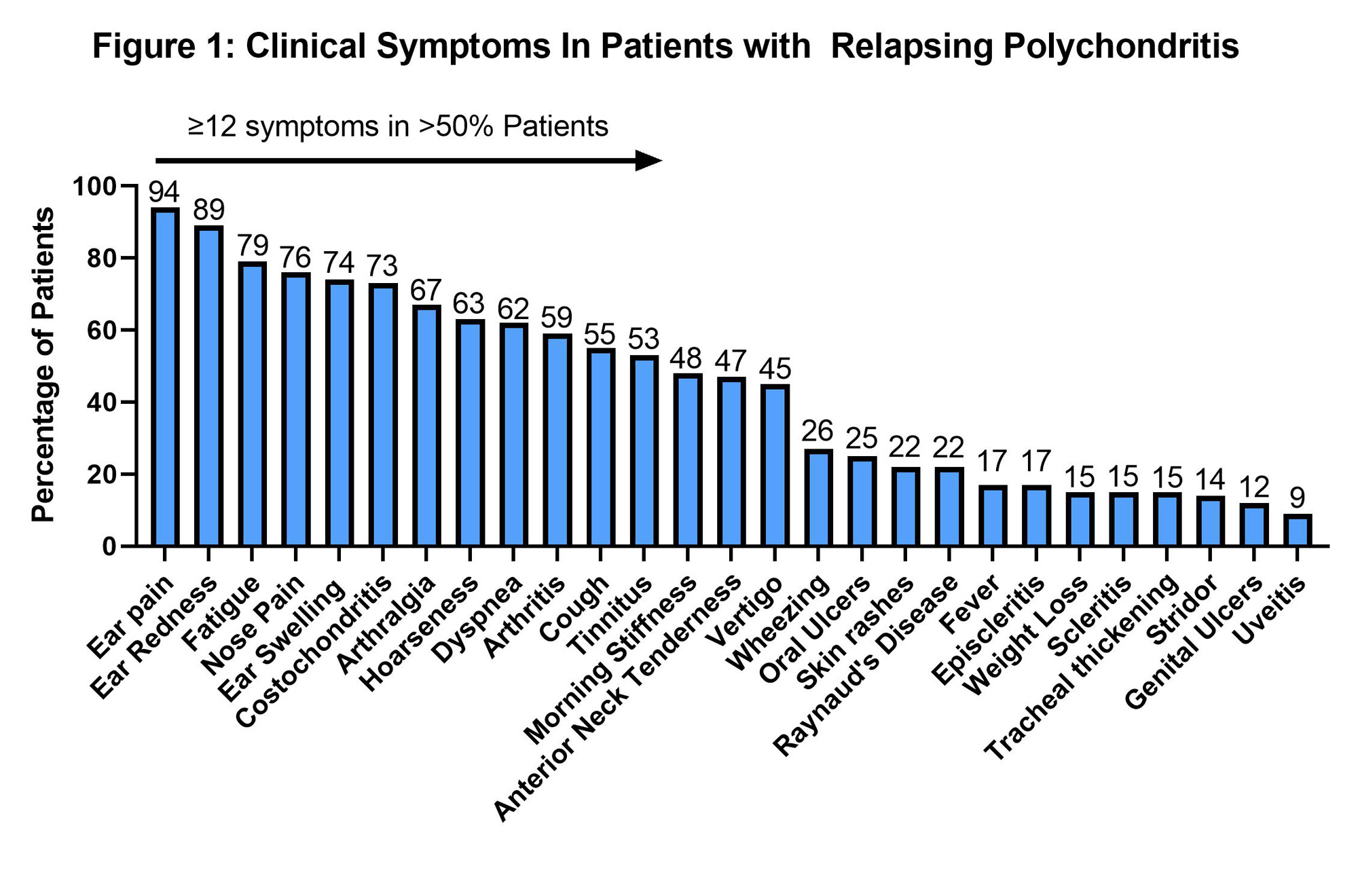
Results: The study included 195 patients with RP. The clinical characteristics are detailed in Figure 1. Mean age was 49 (SD 13) years, with 86% (n=167) female, and 89% (n=174) Caucasians. Mean age at diagnosis was 43 (SD 13) years and mean disease duration was 5 (3-8) years.
All patients in the cohort had ear-nose-throat involvement and the majority (83%, n=163) had musculoskeletal manifestations. Clinical manifestations were heterogeneous and >50% (n=102) of patients had 12 or more symptoms (Figure 1). Permanent organ damage included sensorineural hearing loss in 26% (n=50), auricular deformity in 12% (n=23), saddle nose deformity 12% (n=23) and subglottic stenosis (SGS) in 9% (n=18). In subjects who underwent dynamic CT chest (n=162), tracheomalacia and bronchomalacia were found in 31% (n=50) and 20% (n=32), respectively.
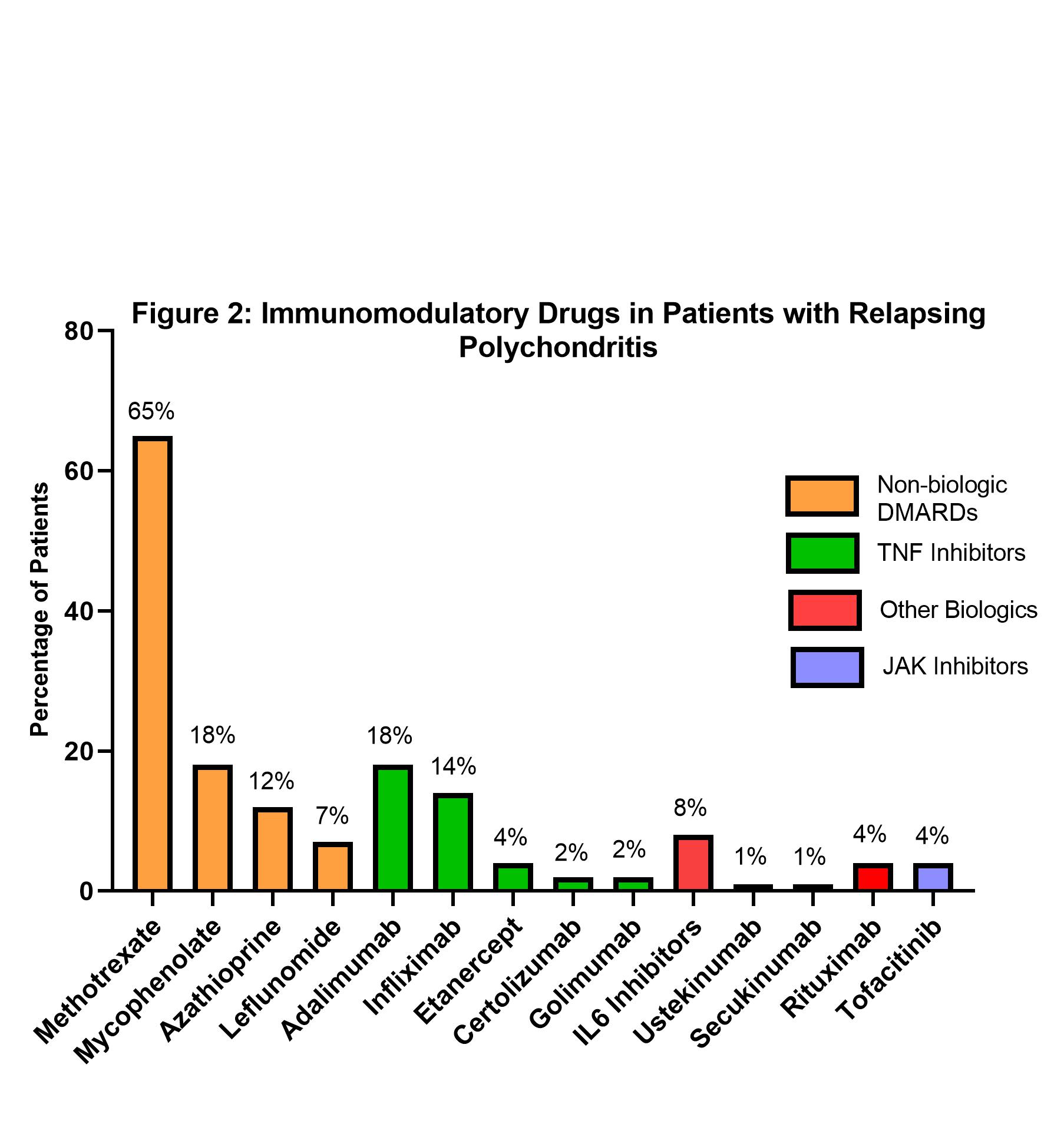
Distribution per treatment group was: group I- 37 (19%) patients; group II- 55 (28%) patients; and group III- 103 (53%) patients. Most patients 95% (n=186) received treatment with GCs. The most frequently prescribed non-biologic drug was methotrexate in 65% (n=126) and the most prescribed class of biologic drugs was TNF inhibitors (29%, n=57) (Figure 2). Patients with arthritis were more likely to be in treatment group III (63%) compared to groups I and II (14% and 22%; P=0.01). Nasal inflammation was common in groups II and III (80% each) compared to group I (56%; P=0.02). Dry cough was associated with Group III (61%) vs Groups I and II (13% and 26%, P= 0.03). Patients with SGS were more likely to be in Group III (88%) vs group I and II (5%, P< 0.01). Tracheal thickening was also more common in Group III (76%) vs Groups I and II (16% and 8%; P=0.03).
Conclusion: Patients with RP have heterogeneous clinical presentations and are treated with a variety of immunosuppressive drugs. Arthritis, nasal inflammation, cough, SGS and tracheal thickening were associated with more frequent use of biologic drugs/JAKi or non-biologic IS. These findings highlight the absence of a consensus approach to treatment of RP and underscore the need for clinical trials and treatment guidelines in RP.
To cite this abstract in AMA style:
Yang R, Rhee R, Quinn K, Amudala N, Grayson P, Merkel P, Ferrada M, Banerjee S. Clinical Manifestations and Immunomodulatory Treatment in Patients with Relapsing Polychondritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-manifestations-and-immunomodulatory-treatment-in-patients-with-relapsing-polychondritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-manifestations-and-immunomodulatory-treatment-in-patients-with-relapsing-polychondritis/