Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with JIA are at high risk for development of chronic anterior uveitis (CAU), impacting 10-20% of this population. Although methotrexate (MTX) is the first-line systemic treatment for JIA-U patients, only 50% respond to therapy. Poor MTX response and delayed escalation of therapy can lead to sight-threatening complications. Non-white race, male sex, ANA positivity, and young age at JIA diagnosis have been associated with a lack of response. We aim to identify clinical risk factors associated with non-response to MTX in JIA-U patients.
Methods: This is a multicenter, prospective study – PEDIA-U and PROMOTE. Patients diagnosed with JIA or JIA-U who were prescribed MTX and who had 12 months follow-up were included. Patients were grouped as MTX non-responders (MTX-NR) vs responders (MTX-R). MTX-NR for CAU was defined as active or worse CAU by Standardization of Uveitis Nomenclature (SUN) criteria, presence of new or worsening complications, and/or need for > 2 drops/day of topical or oral steroids after 3 months. MTX-NR for arthritis was defined as no change in active joint count after 3 months. Clinical phenotypes were compared between MTX-NR and MTX-R using Chi-square and two sample tests. Odds ratios were calculated by multiple logistic regression.
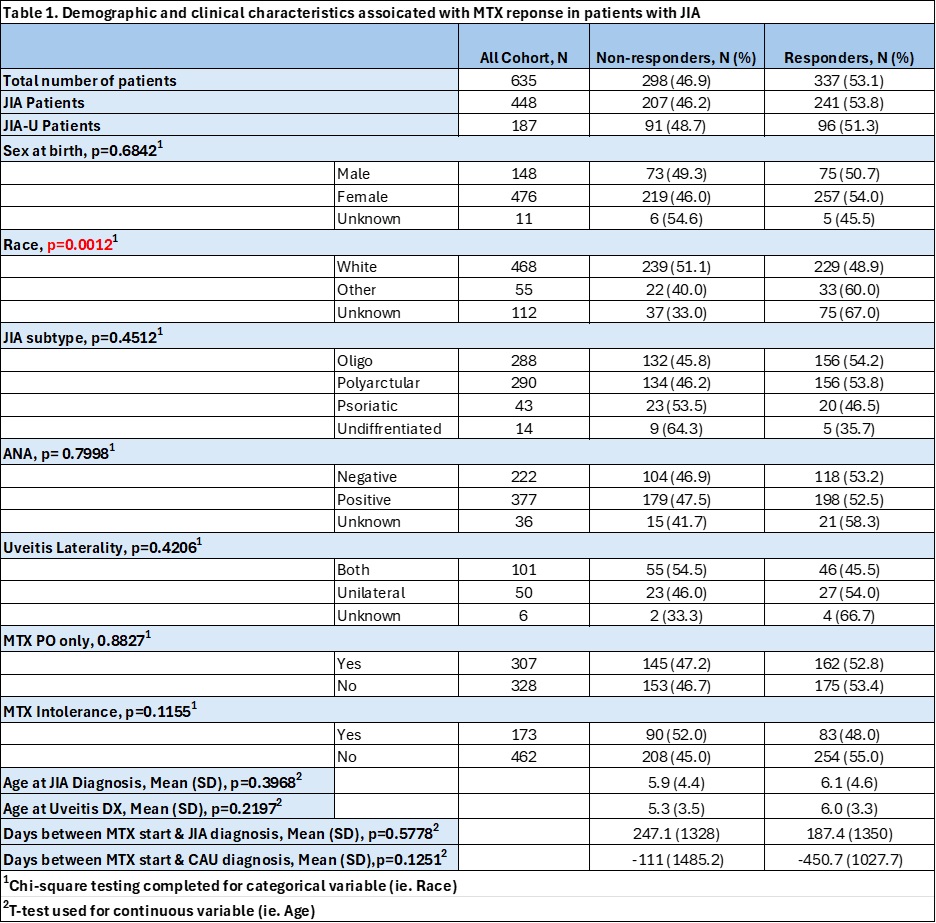
Results: A total of 635 patients with JIA were identified, 187 (29.4%) of whom had JIA-U (Table 1). The overall cohort was predominately white (73.7%) and female (74.9%), with 298 (46.9%) being identified as MTX-NR. Average MTX oral and subcutaneous dose was similar at 16.5 mg/m2 (SD 6.6) and 17.4 mg/m2 (SD 5.7), respectively. White race was associated with MTX-NR on bivariate (X2=20.0, p=0.0012) and multivariate (OR 2.429, CI 1.512-3.904) analysis, with no other significant clinical variables identified. Of the 187 JIA-U patients, 91 (48.6%) were identified as being MTX-NR (Table 2). Of the 91 JIA-U MTX-NR, a majority were non-responders due to arthritis activity (61.5%), followed by both CAU and arthritis activity (26%) and isolated CAU activity (12%). In contrast to the overall cohort, MTX intolerance (MTX-IT) was associated with MTX-NR (X2=9.3, p=0.00023) in the JIA-U group. The most common reason for MTX-IT being GI symptoms (76.2%), followed by laboratory abnormalities (11.9%). The most initiated biological DMARD in MTX-NR patients was adalimumab (37.9%). No significant differences in predictors of response were found between JIA and JIA-U groups across clinical and demographic variables.
Conclusion: Patients of white race and those who experience MTX-IT were significantly more likely to be MTX-NR in the combined and JIA-U cohorts, respectively. Risk factors of MTX-NR from previously reported smaller cohorts were not significant in our population. Our study does not support evidence of using clinical criteria alone to identify at-risk populations for MTX-NR. Future investigation could include ocular findings on novel imaging modalities, biomarkers, and genetics as risk factors of MTX-NR, with the potential for developing individualized JIA-U therapy plans while minimizing ophthalmic morbidity.
To cite this abstract in AMA style:
Jagger A, Becker M, Thompson S, Altaye M, Bohnsack J, Brunner H, Chang M, Cooper A, Davidson S, Duell A, Gangwani B, Langefeld C, Lerman M, Lo M, Pastore S, Pavlenko M, Prahalad S, Quinlan-Waters M, Ramsey L, Schulert G, Simonini G, Stahl E, Stocco G, Sudman M, Taddio A, Miraldi Utz V, Yeung R, Angeles-Han S. Clinical Indicators of Methotrexate Response in Juvenile Idiopathic Arthritis (JIA) and JIA with Uveitis (JIA-U) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/clinical-indicators-of-methotrexate-response-in-juvenile-idiopathic-arthritis-jia-and-jia-with-uveitis-jia-u/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-indicators-of-methotrexate-response-in-juvenile-idiopathic-arthritis-jia-and-jia-with-uveitis-jia-u/

.jpg)