Session Information
Session Type: Abstract Session
Session Time: 4:30PM-5:30PM
Background/Purpose: Cognitive behavioral therapy (CBT) has demonstrated level 1A evidence for management of fibromyalgia (FM). Acceptance and Commitment Therapy (ACT), a form of CBT, has been empirically validated for the management of FM. While these therapies have demonstrated benefit and low risks for FM patients, broad utilization has been hindered due to a lack of access and cost. A prescription digital therapeutic would represent a significant step towards expanding access to ACT for the FM population.An investigational smartphone-based application (FM-ACT) has been developed to deliver self-guided ACT for fibromyalgia management. In this present analysis, the authors assessed the preliminary clinical outcomes of participants using FM-ACT.
Methods: A decentralized, single arm clinical trial (REACT-FM; NCT05011162) is currently ongoing to assess the efficacy, safety, and usability of FM-ACT. Fibromyalgia participants meeting 2016 FM diagnostic criteria received the FM-ACT therapy, which consists of 8 chapters of structured lessons, mindfulness practices, and activities to encourage paced exercise and behavior change.
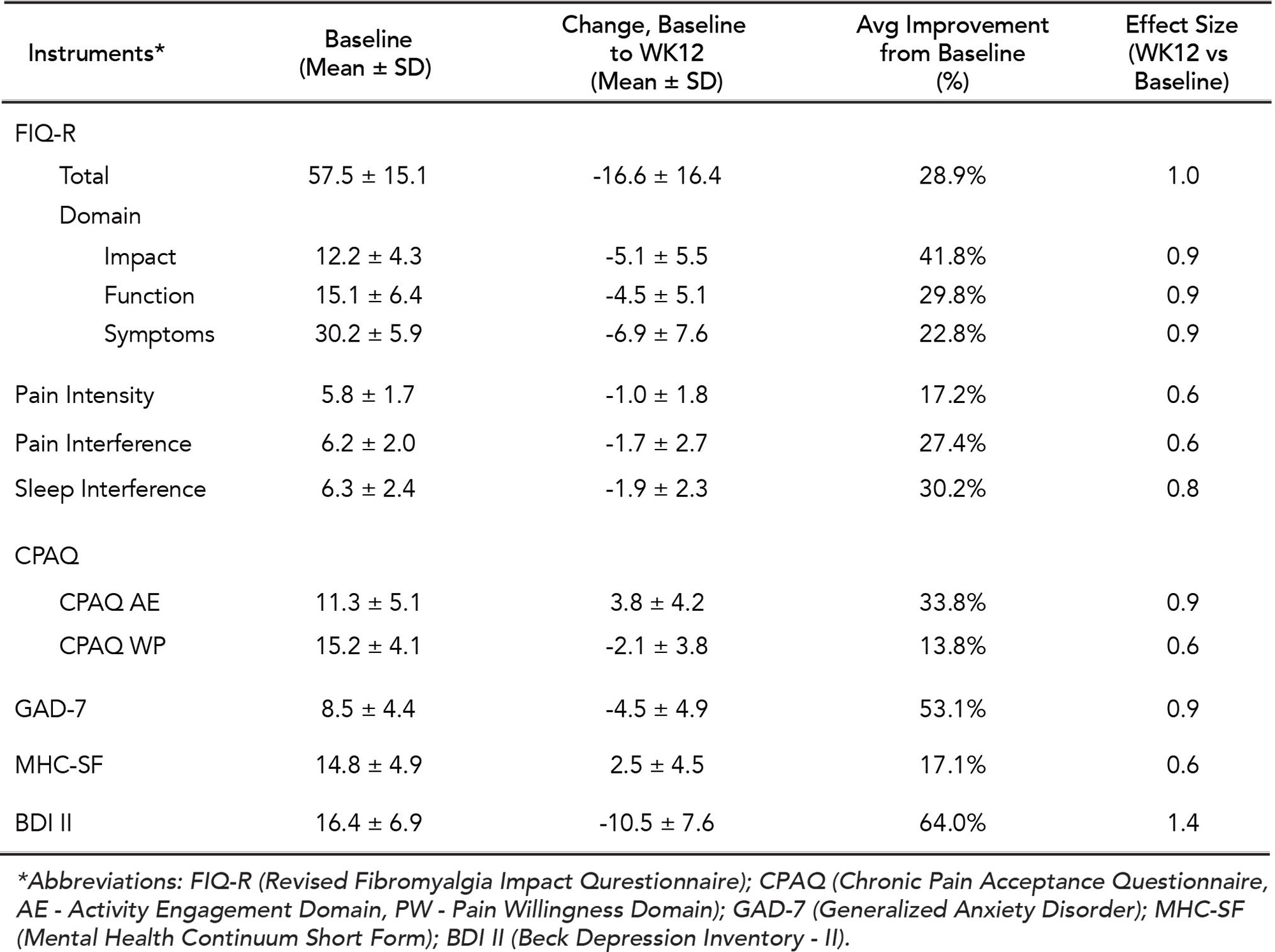
The primary outcome measure is Patient Global Impression of Change (PGIC). Other outcomes collected include the Revised Fibromyalgia Impact Questionnaire (FIQ-R), pain intensity and interference, sleep interference, depression, anxiety, pain acceptance, mindfulness, and quality of life (QoL). Outcomes at week 12 compared to baseline were analyzed for all completed participants.
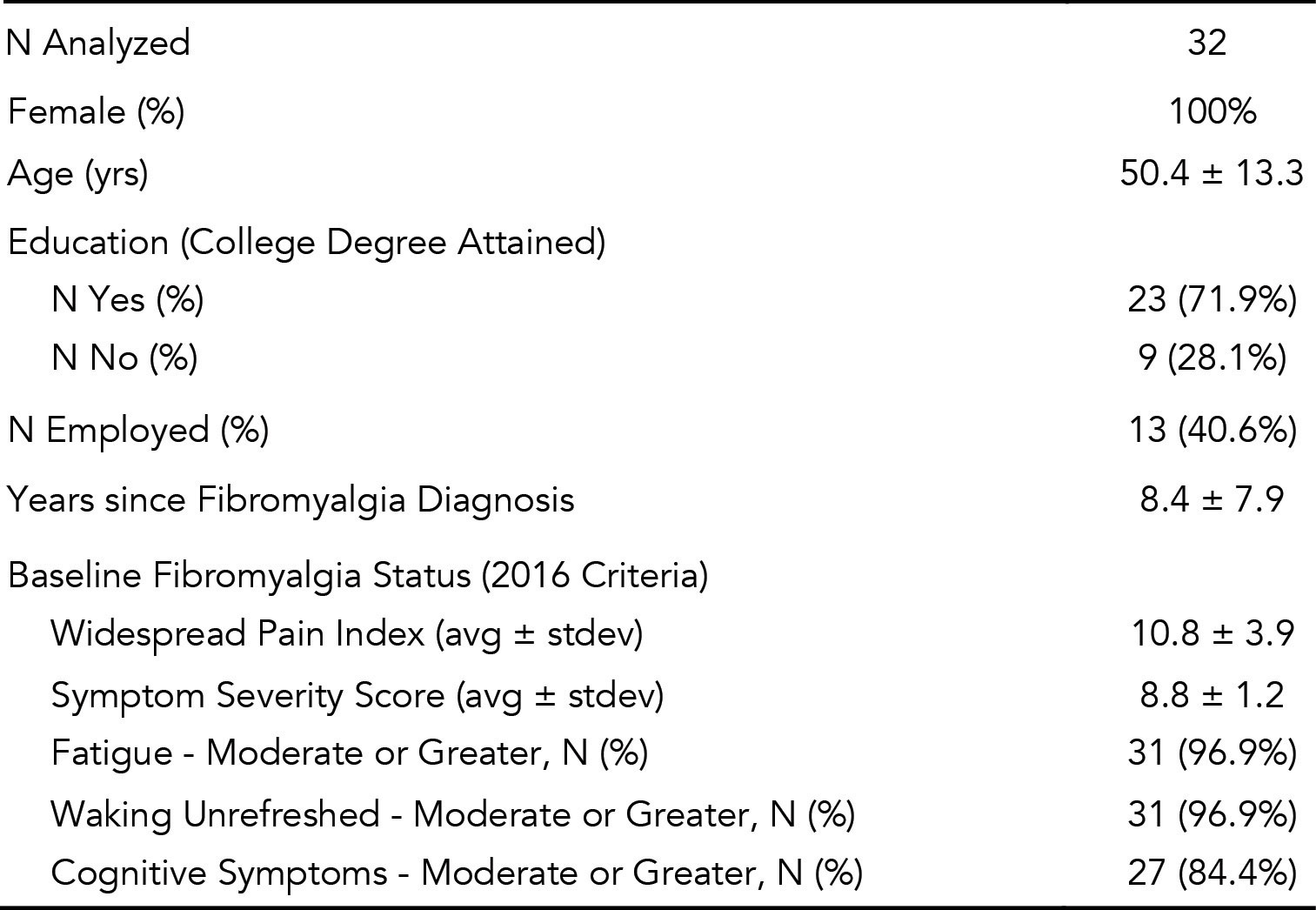
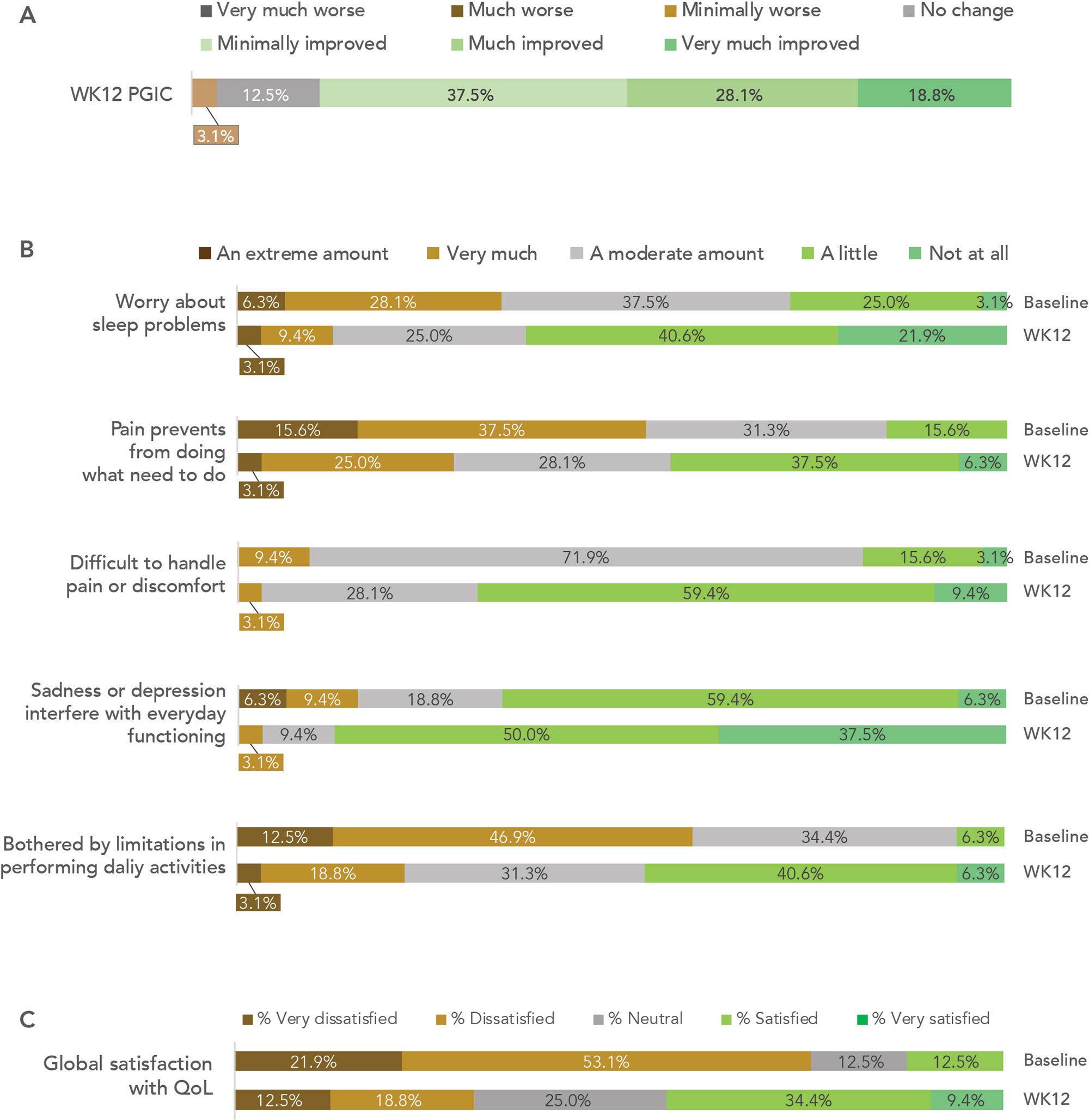
Results: Forty participants were eligible at the time of analysis, among whom 32 had completed week 12 ePROs (completer analysis, Table 1). On PGIC, 84% of participants reported improvement in their FM, with 47% reporting to be much or very much improved (Fig 1A). Significant improvements (p values < 0.01) with medium to large effect sizes were observed across all outcome measures at week 12 compared to the baseline (Table 2). Improvement of ≥ 20% in FIQR total score was observed in 66% of participants. Compared to baseline, 22-50% more participants achieved positive QoL outcomes at week 12 (Fig 1B), with 31% more reporting satisfaction with their global QoL (Fig 1C). No device or study procedure related adverse events were reported.
Conclusion: This study reported early results from a prospective single-arm clinical trial evaluating a smartphone based digital therapeutic for FM management. The data reinforced the positive outcomes observed from a previous RCT (Catella et al. ACR Convergence 2021). At the end of the 12-week therapy, participants achieved significant improvement in all areas measured, including pain severity and interference, sleep interference, and psychological well-being. Furthermore, meaningful improvement in QoL was observed, exhibited by substantial increases in global satisfaction with QoL as well as increases in participants who were able to manage their pain, sleep, and daily function.
Study limitations included a small cohort and the lack of a comparator arm. These initial results require further confirmation as the present trial progresses. An additional RCT is currently underway (PROSPER-FM, NCT05243511).
*Questions were selected from World Health Organization Quality of Life instrument (WHOQOL_100).
To cite this abstract in AMA style:
Dai Y, Rosenbluth M, Gendreau M, Vega N, Ghalib Z, Kraus A, Keefe B. Clinical Impact of a Digital Behavioral Therapy for Fibromyalgia Management in a Decentralized Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/clinical-impact-of-a-digital-behavioral-therapy-for-fibromyalgia-management-in-a-decentralized-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-impact-of-a-digital-behavioral-therapy-for-fibromyalgia-management-in-a-decentralized-trial/