Session Information
Date: Saturday, November 6, 2021
Title: Fibromyalgia & Other Clinical Pain Syndromes Poster (0118–0127)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Recommendations for fibromyalgia management include both pharmacologic and nonpharmacologic treatments. Cognitive behavioral therapy (CBT) has demonstrated level 1A evidence for fibromyalgia management, though access is limited due to few trained clinicians and cost. An at home digital behavioral therapy could significantly increase access.
A smartphone-based Acceptance and Commitment Therapy (ACT) program was developed for fibromyalgia management. ACT is a modern type of CBT increasingly used to manage chronic pain that focuses on acceptance of uncontrollable and untreatable symptoms. A multi-site study is underway to understand the feasibility of a largely virtual study and determine the impact of smartphone-delivered ACT compared to a digital active control.
Methods: Participants diagnosed with fibromyalgia using 2016 criteria were randomized to digital ACT or an active control. Participants remained stable on other ongoing fibromyalgia treatment. The digital ACT intervention consists of 41 daily sessions of structured ACT lessons, mindfulness practices, and activities to encourage paced exercise and behavior change. An active control was implemented to account for study engagement and expectation biases. Components of the active control (Symptom Tracker, or ST) include daily symptom tracking and monitoring and access to patient education. During the 12 week study, participants were expected to use the smartphone applications at least 5 days a week.
FIQ-R, the primary outcome, was administered weekly. Secondary outcomes include PGIC, weekly recall pain intensity and interference, and sleep quality (NRS), BDI-II, PROMIS-29, Five Facets of Mindfulness, and Psychological Inflexibility in Pain Scale.
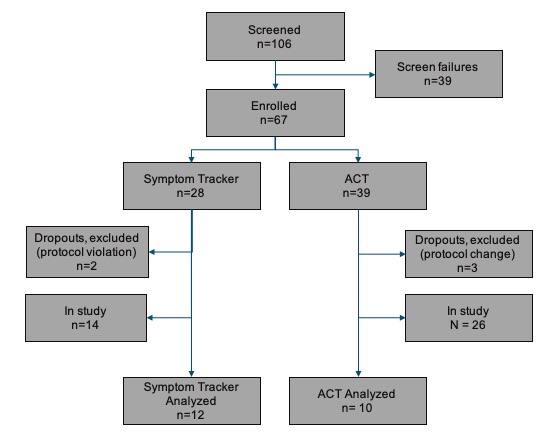
The study has enrolled 67 participants. Twenty-seven participants have completed the study to date and 22 are included in the current analysis set (Figure 1).
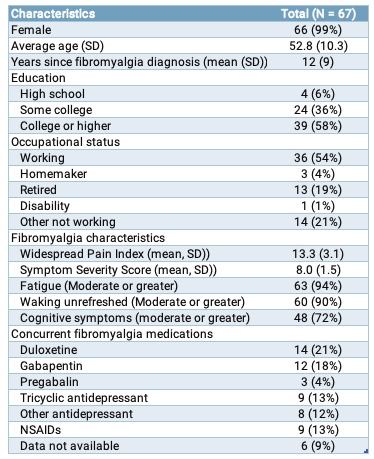
Results: The characteristics of the participants are summarized in Table 1. Engagement in both arms is high throughout the study (an average completion of 5 sessions per week in each arm).
Improvement of 20% or greater in total FIQR score was observed in 50% of the ACT group compared to 25% in the ST group. Participants in ACT improved on average 10.5 points (20%) in total FIQR score compared to 4.8 points (9%) in ST. Forty percent of ACT participants reported “much improved” or greater on the PGIC compared to 0% in ST. More improvement was seen in ACT over ST in most other outcome measures. No treatment related adverse events have been observed. This study is ongoing and full results are expected this summer.
Conclusion: A mostly virtual digital therapy study is feasible with high engagement in both ACT and the active control. Participants in the ACT arm achieved greater reductions on FIQ-R and showed greater improvement on PGIC compared to ST. Participants in the ACT arm showed greater improvement relative to the ST arm on ACT process measures, suggesting digital ACT is effectively targeting therapeutic processes of change.
The findings suggest that digital ACT may improve fibromyalgia management and appears to be well tolerated.
To cite this abstract in AMA style:
Catella S, Gendreau M, Vega N, Kraus A, Rosenbluth M, Soefje S, Malhotra S, Arnold L. Clinical Impact of a Digital Behavioral Therapy for Fibromyalgia Management: A Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/clinical-impact-of-a-digital-behavioral-therapy-for-fibromyalgia-management-a-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-impact-of-a-digital-behavioral-therapy-for-fibromyalgia-management-a-randomized-controlled-trial/