Session Information
Date: Saturday, November 16, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: To investigate clinical features of SpA patients with and without IBD, identifying the patient group that would benefit from initiating therapy with efficacy in both SpA and IBD at the time of diagnosis of either entity.
Methods: Methods
The METEOR SpA database encompasses patients with a clinical diagnosis of SpA, exhibiting peripheral or axial disease. These patients were stratified based on the presence or absence of concomitant IBD, reported by the local investigator. Patient and disease characteristics, patient and physician reported outcomes, disease activity measures, physical functioning, prescribed treatment and local imaging assessments were collected. Presence of IBD was reported as current or past and was likewise used for the patient stratification. Comparisons of continuous outcomes between two groups were performed using Student’s t–test for independent samples or the Mann-Whitney U test (according to the distribution of data).
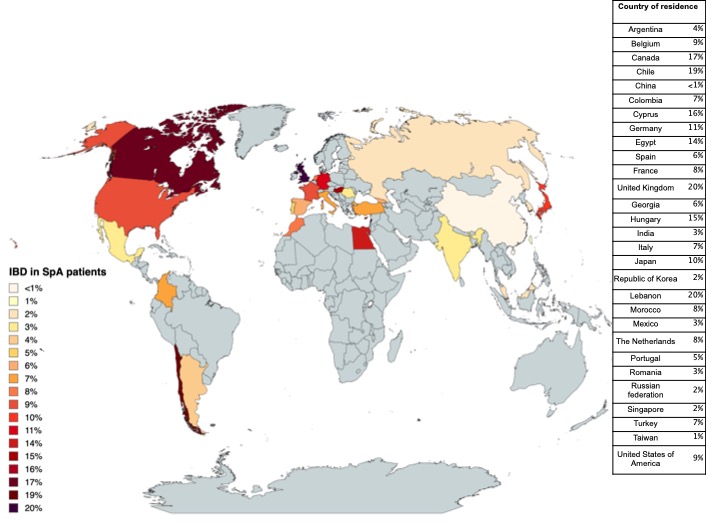
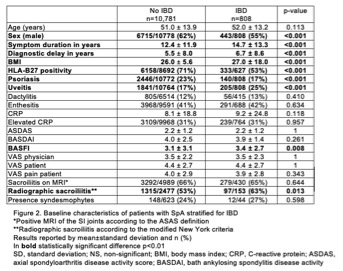
Results: We identified 11,589 SpA patients with available data on IBD, of which 7% of patients had concomitant IBD with mean symptom duration of 12±12 years at inclusion in METEOR. Prevalence ranged from < 1% to 20% across 29 countries worldwide (fig 1). In SpA patients with IBD, male predominance was less pronounced, with marginally higher BMI versus those with SpA only (fig 2). Moreover, a marginally longer disease duration and diagnostic delay was observed. Lower prevalence of HLA-B27 and psoriasis was seen in the presence of IBD, even though uveitis rates were found to be higher. No differences were noted regarding the prevalence of dactylitis, enthesitis or elevated CRP. Patients showed similar disease activity, reflected by BASDAI, ASDAS and both physician and patient VAS.
SpA patients with IBD seemed to exhibit more structural damage of the sacroiliac (SI) joints, as indicated by modified NY criteria, compared to those without IBD. Similarly, higher BASFI was reported. However, the presence of inflammatory lesions on MRI (ASAS definition), as well as the presence of syndesmophytes was found to be similar among groups, although data on the latter was scarce.
Conclusion: In the METEOR database, SpA patients with IBD, as opposed to those without IBD, were less frequently male and more frequently HLA-B27 negative, yet demonstrated higher rates of uveitis and lower rates of psoriasis. These results support the lower rates of HLA-B27 and psoriasis described in Belgian SpA patients with IBD. [1] Despite the limitations of the interpretation of structural damage on conventional radiograph, SpA patients with concomitant IBD exhibited more structural damage on SI joints compared to patients without IBD, possibly mirroring gut inflammation as a risk factor for evolution into radiographic axSpA. Nevertheless, these patients also exhibited longer symptom duration. In light of evolving, occasionally more selective, therapeutic agents, identifying SpA patients at risk of developing IBD at the time of diagnosis would prevent unnecessary therapy cycling. Further research should focus on identifying baseline risk factors in large prospective studies.
To cite this abstract in AMA style:
de hOoge M, Machado P, Bergstra S, Dougados M, López Medina C, Navarro Compán V, Benavent D, Poddubnyy D, kilic L, Carron P, Elewaut D, Van den Bosch F, Varkas G. Clinical Features of Patients with SpA, with or Without IBD: Results from the METEOR Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clinical-features-of-patients-with-spa-with-or-without-ibd-results-from-the-meteor-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-features-of-patients-with-spa-with-or-without-ibd-results-from-the-meteor-cohort/