Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients (pts) in PsA clinical trials often have greater joint involvement than in the clinical setting due to trial inclusion criteria,1 thus it is important to assess treatment efficacy in pts with varying degrees of joint involvement. We investigated the efficacy of bimekizumab (BKZ), a selective inhibitor of interleukin (IL)-17F in addition to IL-17A, in subgroups of pts with varying joint involvement at baseline (BL), using data pooled from two phase 3 studies.
Methods: Post hoc analysis assessed subcutaneous BKZ 160 mg every 4 weeks (wks; Q4W) in pts with PsA and varying BL joint involvement. Pts were grouped by BL swollen joint count (SJC); thresholds defined based on quartile boundaries at BL (SJC ≤5 / 6–≤7 / 8–≤12 / >12). Due to small pt numbers with 3/4 SJC at BL, pts with 5 SJC were included in the first quartile; pt numbers in each quartile therefore vary.Pts were pooled from the BE OPTIMAL (NCT03895203; biologic DMARD [bDMARD]-naïve) and BE COMPLETE (NCT03896581; TNF inhibitor inadequate response/intolerance [TNFi-IR]) trials. As trials were different sizes (BE OPTIMAL 431 BKZ, 281 PBO; BE COMPLETE 267 BKZ, 133 PBO), proportions of bDMARD-naïve and TNFi-IR pts are not equal per quartile. Inclusion criteria for each trial included ≥3 tender and swollen joints. Both had a 16-wk double-blind, placebo (PBO)-controlled period. Pts completing Wk 52 of BE OPTIMAL or Wk 16 of BE COMPLETE could enter BE VITAL (NCT04009499; open-label extension), in which all pts received BKZ. BE OPTIMAL included a reference arm (adalimumab 40 mg Q2W); data not reported due to small pt numbers in SJC quartiles.Outcomes reported: ACR ≥50% improvement, Psoriasis Area and Severity Index 100% improvement, minimal disease activity, SJC=0, Pain visual analog scale ≥50/70% improvement from BL, Health Assessment Questionnaire – Disability Index minimal clinically important difference (≥0.35 decrease from BL in pts with BL score ≥0.35). Data are pooled across both trials (non-responder imputation; observed case). Data are reported by randomization group at Wk 16, Year 1 (Wk 52), and Year 2 (pooled Wk 104/100 from BE OPTIMAL/BE COMPLETE).
Results: At BL, 370 pts had SJC ≤5, 215 had 6–≤7, 275 had 8–≤12, and 252 had >12. Demographic and disease characteristics by BL SJC are presented in the Table.At Wk 16, BKZ-treated pts demonstrated numerically greater improvements in joint, skin, composite, and patient-reported outcomes vs PBO, across pts with varying BL joint involvement. For all domains assessed, improvements were sustained to 2 years in BKZ-randomized pts, with robust efficacy across pts with varying BL joint involvement (Figure 1–2). For pts who switched from PBO to BKZ at Wk 16, improvements in efficacy similar to BKZ-randomized pts with varying BL joint involvement were reported to Year 1 and sustained to Year 2.
Conclusion: In pts with PsA and varying BL joint involvement, BKZ treatment demonstrated greater improvements vs PBO across disease domains at Wk 16, and improvements were sustained to 2 years. Robust efficacy was observed across all groups of pts with varying BL joint involvement, reflecting the broader range of pts seen in clinical practice.References: 1. Alle G. J Rheum 2025;52:138–44.
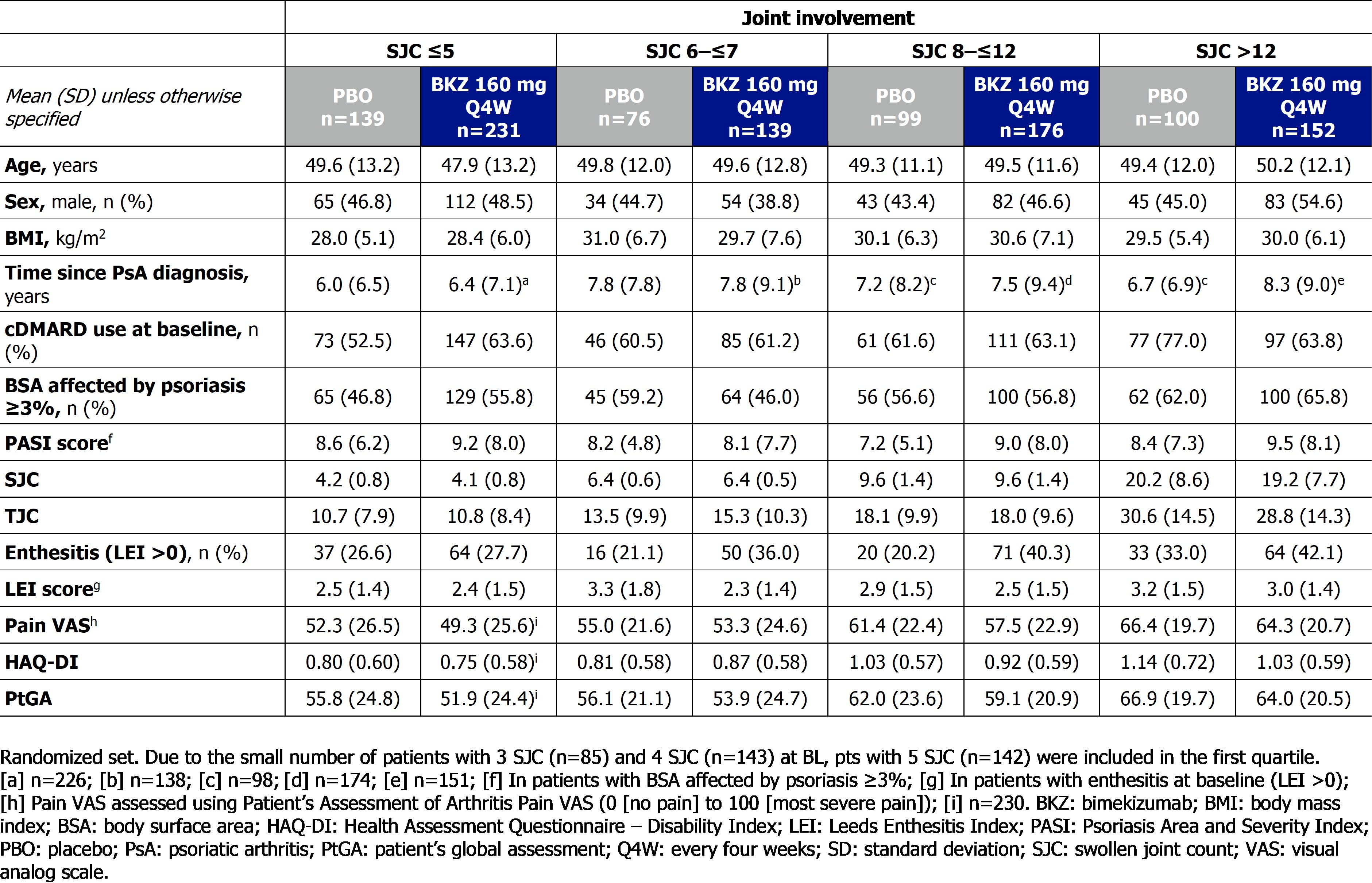 Table. Baseline demographics and disease characteristics, reported by baseline joint involvement
Table. Baseline demographics and disease characteristics, reported by baseline joint involvement
.jpg) Figure 1. (A) ACR50, (B) PASI100, (C) MDA, and (D) SJC=0 responders at Week 16, Year 1, and Year 2, reported by baseline joint involvement (NRI, OC)
Figure 1. (A) ACR50, (B) PASI100, (C) MDA, and (D) SJC=0 responders at Week 16, Year 1, and Year 2, reported by baseline joint involvement (NRI, OC)
.jpg) Figure 2. (A) Pain50, (B) Pain70, and (C) HAQ-DI MCID responders at Week 16, Year 1, and Year 2, reported by baseline joint involvement (NRI, OC)
Figure 2. (A) Pain50, (B) Pain70, and (C) HAQ-DI MCID responders at Week 16, Year 1, and Year 2, reported by baseline joint involvement (NRI, OC)
To cite this abstract in AMA style:
Mease P, Gossec L, Kameda H, Nicholls D, Rahman P, Ink B, Bajracharya R, Healy P, Coates L. Clinical Efficacy of Bimekizumab Treatment up to 2 Years Across Patients with Psoriatic Arthritis and Varying Baseline Joint Involvement: Results from a Post Hoc Analysis of Two Pooled Phase 3 Studies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/clinical-efficacy-of-bimekizumab-treatment-up-to-2-years-across-patients-with-psoriatic-arthritis-and-varying-baseline-joint-involvement-results-from-a-post-hoc-analysis-of-two-pooled-phase-3-studies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-efficacy-of-bimekizumab-treatment-up-to-2-years-across-patients-with-psoriatic-arthritis-and-varying-baseline-joint-involvement-results-from-a-post-hoc-analysis-of-two-pooled-phase-3-studies/
