Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: GALAXI 1 is a Phase 2, double-blind, placebo (PBO)-controlled, multicenter study evaluating the efficacy/safety of guselkumab (GUS), a selective IL-23 p19 antagonist, in patients with moderately to severely active Crohn’s disease (CD) with inadequate response/intolerance to conventional therapies (corticosteroids, immunomodulators) and/or biologics (tumor necrosis factor antagonists, vedolizumab). At week (Wk) 12, all GUS induction doses (200, 600, and 1200 mg intravenous [IV]) had greater improvements vs PBO for key clinical/endoscopic outcomes. We report the clinical efficacy and safety of maintenance treatment through Wk 48.
Methods: GALAXI employed a treat-through design over 48 wks. In induction, patients were randomized to GUS 200, 600, or 1200 mg IV, ustekinumab (UST) ~6 mg/kg IV, or PBO IV. Patients transitioned to maintenance dosing as follows: PBO non-responders→UST ~6 mg/kg IV→90 mg subcutaneous (SC) every 8 weeks (q8w); PBO responders→PBO SC q4w, GUS 200 mg IV→100 mg SC q8w, GUS 600 mg IV→200 mg SC q4w, GUS 1200 mg IV→200 mg SC q4w, and UST ~6 mg/kg IV→90 mg SC q8w. Patients randomized to PBO were not included in the Wk 48 efficacy analyses. Primary and major secondary endpoints evaluated the efficacy of GUS vs PBO at Wk 12. Evaluations of Wk 48 endpoints were prespecified but not multiplicity controlled. UST was a reference arm; the study was not powered to evaluate differences between treatment groups with respect to efficacy at Wk 48.
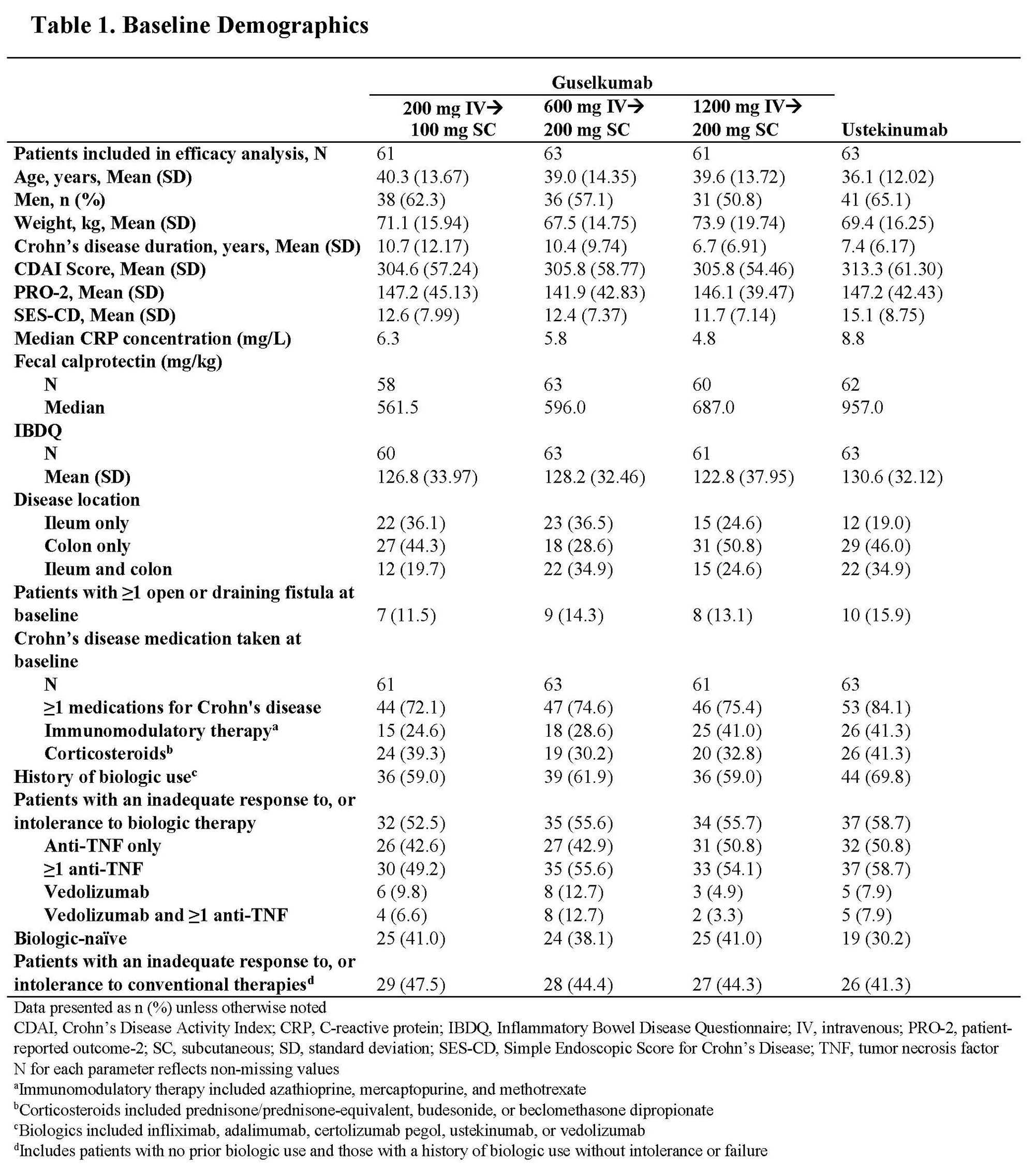
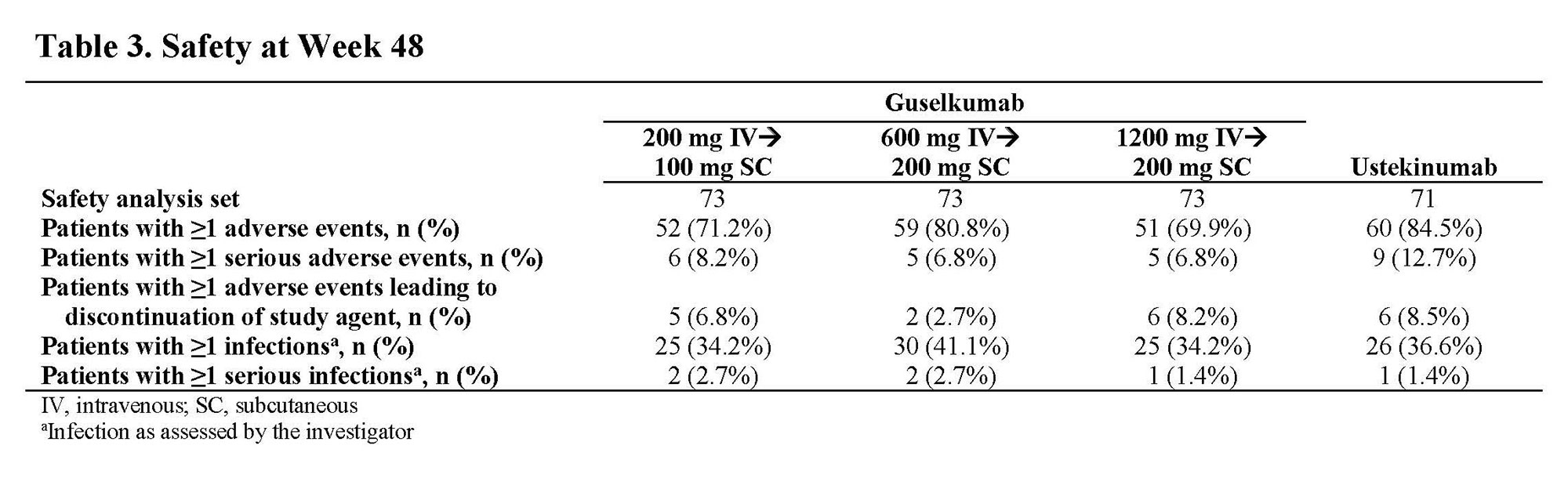
Results: Through Wk 48, 248 patients in the primary efficacy analysis set were randomized and evaluated. Baseline demographics were similar across groups (Table 1). Discontinuation rates were low across active treatment groups. No dose response was observed across clinical efficacy assessments (Table 2). Proportions of patients achieving clinical remission at Wk 48 ranged from 57.4% to 73.0% among GUS dose groups. The vast majority of patients in clinical remission were also in corticosteroid-free remission at Wk 48, with rates ranging from 55.7% to 71.4% among GUS dose groups. Patient-reported outcome (PRO)-2 remission rates ranged from 50.8% to 69.8%, and proportions of patients achieving clinical response ranged from 67.2% to 84.1% among GUS dose groups. Proportions of patients achieving abdominal pain scores ≤1 or daily average number of liquid or very soft stools ≤3 are presented in Table 2. Outcomes in the reference UST group are also shown in Table 2. Key safety event rates were similar among the GUS dose groups (Table 3); no opportunistic infections, cases of tuberculosis, or deaths were reported in any group.
Conclusion: In this treat-through Phase 2 study of patients with moderately to severely active CD, GUS was safe and effective. GUS induction followed by SC maintenance achieved high rates of clinical efficacy at Wk 48. Safety results were consistent with the known safety profile in approved indications.
To cite this abstract in AMA style:
Danese S, Panaccione R, Rubin D, Sands B, Reinisch W, D’Haens G, Panés J, Gonzalez S, Weisel K, Sahoo A, Frustaci M, Yang Z, Sandborn W, Afzali A, Hisamatsu T, Andrews J, Feagan B. Clinical Efficacy and Safety of Guselkumab Maintenance Therapy in Patients with Moderately to Severely Active Crohn’s Disease: Week 48 Analyses from the Phase 2 GALAXI 1 Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/clinical-efficacy-and-safety-of-guselkumab-maintenance-therapy-in-patients-with-moderately-to-severely-active-crohns-disease-week-48-analyses-from-the-phase-2-galaxi-1-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-efficacy-and-safety-of-guselkumab-maintenance-therapy-in-patients-with-moderately-to-severely-active-crohns-disease-week-48-analyses-from-the-phase-2-galaxi-1-study/