Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Gastrointestinal (GI) tract involvement is the most common of all internal organ involvement in systemic sclerosis (SSc). The University of California at Los Angeles, Scleroderma Clinical Trial Consortium, Gastrointestinal Tract Instrument 2.0 (UCLA GIT 2.0) is validated to capture GI morbidity in patients with SSc (1). However, the routine clinical investigation of GI involvement in these patients is not standardized and there is no consensus about when and how frequently an esophago-gastro-duodenoscopy (EGD) should be performed.
The aims of this study were to determine in an unselected, real-life cohort of patients with SSc, 1) if the UCLA GIT 2.0 could discriminate patients for whom a rheumatologist with experience in SSc would recommend an EGD, and 2) if the UCLA GIT 2.0 could identify patients with endoscopic esophagitis.
Methods: We selected patients fulfilling the ACR/EULAR 2013 criteria for SSc from the Zurich EUSTAR cohort, having completed at least once the UCLA GIT 2.0 questionnaire at an EUSTAR visit. We reviewed the medical charts of the selected patients from 2013 to 2019 and recorded data on EGD performed in an interval of ±3 months from a EUSTAR visit. We analyzed by general linear mixed effect models (GLMM) several parameters, including UCLA GIT 2.0 and its subscales, considered as potentially associated with 1) the referral to EGD and 2) endoscopic esophagitis.
Results: We identified 346 patients (82.7% female, median age 63 years, median disease duration 10 years, 23% with diffuse cutaneous SSc) satisfying the inclusion criteria, who filled in 940 UCLA GIT 2.0 questionnaires.
For the first objective, we excluded 31/940 visits because EGD was done shortly (< 3 months) before the EUSTAR visit. In the 909 remaining visits, EGD was recommended by the expert rheumatologists in 128 cases. The UCLA GIT 2.0 total score and some of its subscales (reflux, distention/bloating, social functioning score), but also the modified Rodnan skin score (mRSS) and esophageal and stomach symptoms by medical history were associated with the referral to EGD (Table 1).
For the second objective, we identified 177 EGD performed in 145 patients. In GLMM, esophageal symptoms and, to a lesser extent, mRSS, correlated with endoscopic esophagitis, while neither the total ULCA GIT 2.0 score nor any of its subscales showed any association with this finding (Table 2).
Conclusion: In a real-life setting, UCLA GIT 2.0 subscales (reflux, distention/bloating, social functioning) and total score strongly associated with expert interpretation of gastroesophageal symptoms and consecutive referral to EGD. However, they showed no correlation with esophagitis on EGD. The main clinical association of esophagitis was the presence of esophageal symptoms.
Reference: (1) Khanna D, et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum. 2009;61(9):1257-63.
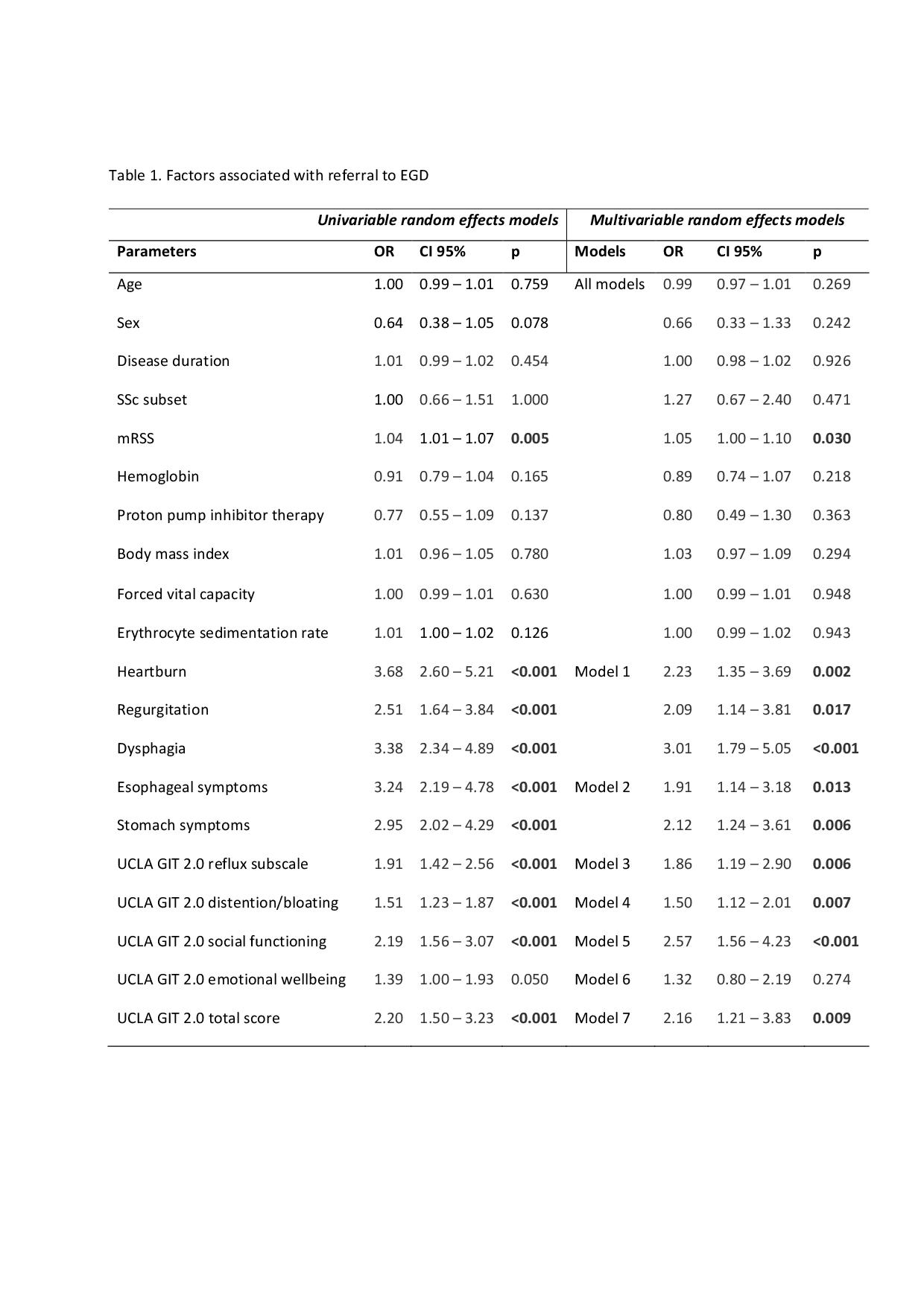 Table 1. Factors associated with referral to EGD
Table 1. Factors associated with referral to EGD
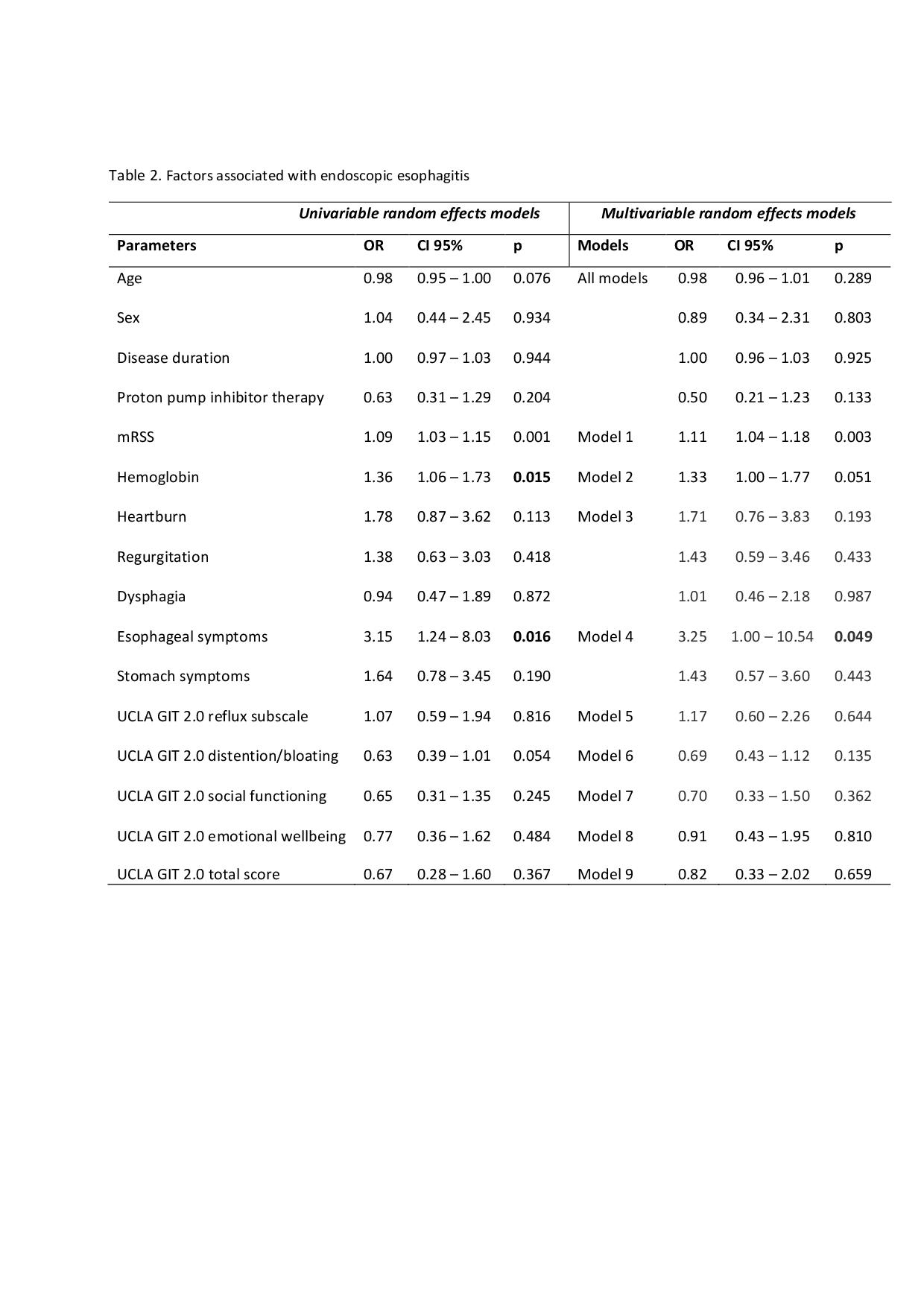 Table 2. Factors associated with endoscopic esophagitis
Table 2. Factors associated with endoscopic esophagitis
To cite this abstract in AMA style:
Zampatti N, Garaiman A, Jordan S, Maurer B, Dobrota R, Becker M, Distler O, Mihai C. Clinical Correlates and Relevance of the UCLA GIT 2.0 Instrument for Indication for Esophagogastroduodenoscopy and Endoscopic Esophagitis in Real-life Patients with Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/clinical-correlates-and-relevance-of-the-ucla-git-2-0-instrument-for-indication-for-esophagogastroduodenoscopy-and-endoscopic-esophagitis-in-real-life-patients-with-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-correlates-and-relevance-of-the-ucla-git-2-0-instrument-for-indication-for-esophagogastroduodenoscopy-and-endoscopic-esophagitis-in-real-life-patients-with-systemic-sclerosis/
