Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Antinuclear antibodies (ANAs) are a diverse group of autoantibodies that are commonly present in SLE and other autoimmune (AI) disorders. However, a positive ANA test also occurs in 12-20% of the general population, and 2% of these are at high titers. A high-titer ANA is generally interpreted as being more likely to indicate AI disease; however, the significance of a high-titer ANA test in individuals without an AI disease is not known. We tested the hypothesis that the presence of a high ANA titer in people without AI disease represents a state of immune dysregulation that is associated with altered risk for disease.
Methods: To define the clinical impact of a high ANA titer (ANA≥1:640) in people without AI disease, we conducted a retrospective case-control study using the Vanderbilt University Medical Center’s de-identified electronic medical record (EMR) system. Individuals ≥18 years old with an ANA test performed as part of their clinical care were studied. Individuals with a diagnostic code for any AI disease known to be associated with ANA+ (such as SLE, RA, autoimmune hepatitis, etc.) were excluded. Individuals were classified into two exclusive groups based on ANA results: high titer (HT, ANA≥1:640) and low titer (LT, ANA≤1:80). The prevalence of clinical diagnoses recorded in the EMR within (±) 90 days of the ANA test were compared between the groups using a phenome-wide association study (PheWAS) approach. For each condition: cases were participants with ≥2 phecodes, controls were those without a closely related phecode, and individuals with only 1 phecode were excluded. Phecodes with fewer than 100 cases were excluded to assure power. Analyses were adjusted for age at ANA testing, sex, median body mass index (BMI), and reported race. A P-value < 1E-05 was considered significant.
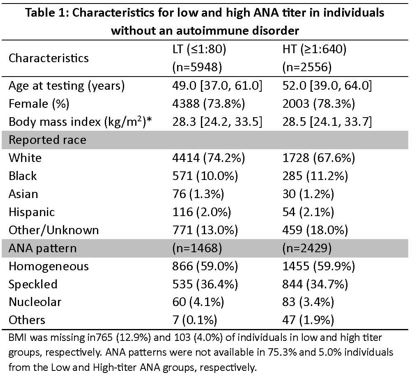
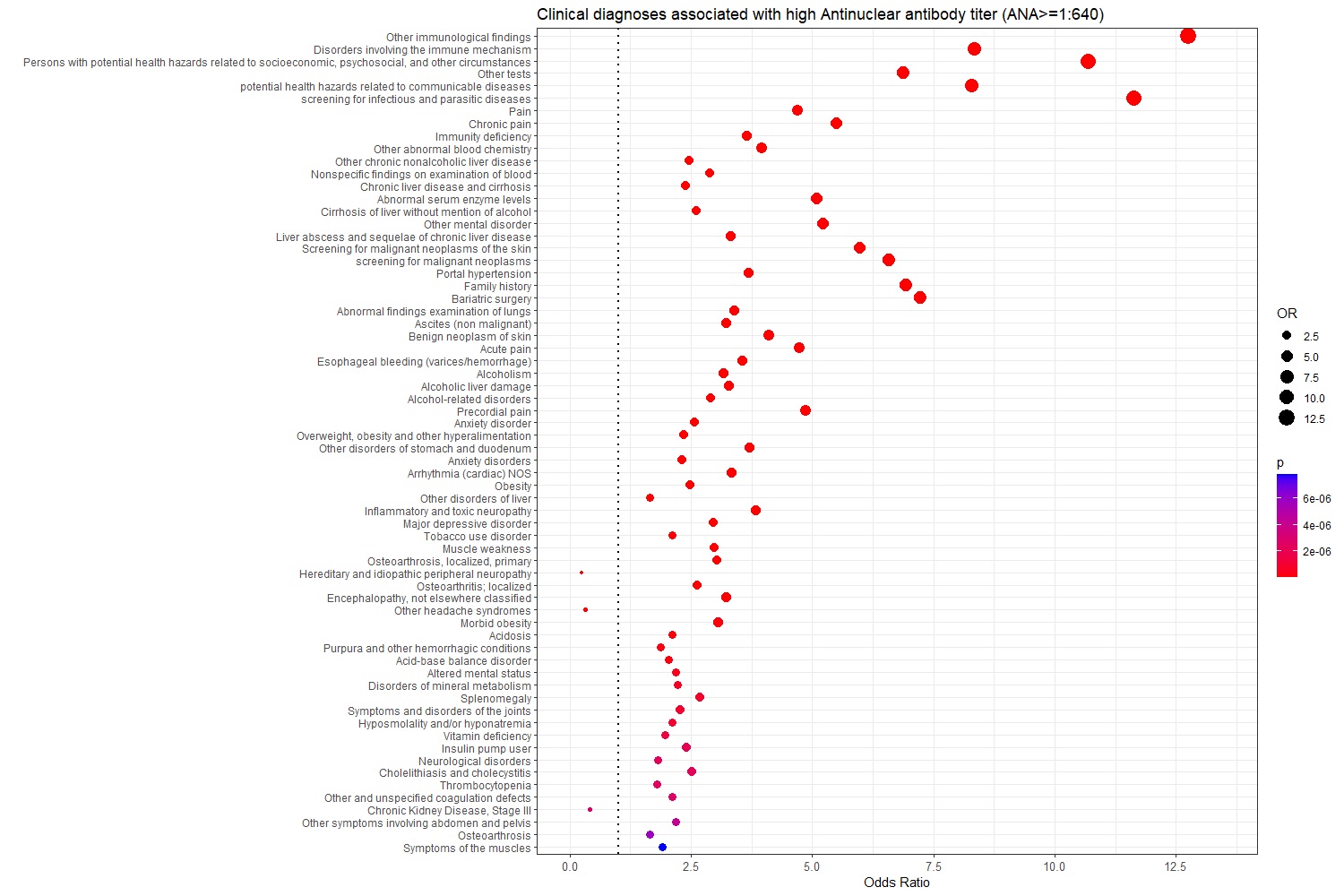
Results: There were 5948 individuals in the LT group and 2556 in the HT group. Table 1 shows age, sex, race, BMI, and ANA pattern distribution among groups. Sixty-six clinical diagnoses were significantly associated with a high ANA titer. The most significant associations with high-titer ANA were with “Other immunological findings” (P=8.3E-236) and with “Disorders involving immune mechanism” (P=8.0E-223); phecodes that reflect ICD coding for an abnormal immunological test. A high ANA titer was strikingly associated with increased risk of diagnoses related to alcoholic and non-alcoholic liver disease and its complications (Figure 1).
Conclusion: A high ANA titer in the absence of an autoimmune disease was associated with increased risk of liver-related disorders and decreased risk of renal-related disorders.
To cite this abstract in AMA style:
Chung M, Shelley J, Still J, Karakoc G, Ruan X, Mosley J, Stein C, Kawai V. Clinical Conditions Associated with Presence of a High-titer Antinuclear Antibody in Individuals Without Autoimmune Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clinical-conditions-associated-with-presence-of-a-high-titer-antinuclear-antibody-in-individuals-without-autoimmune-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-conditions-associated-with-presence-of-a-high-titer-antinuclear-antibody-in-individuals-without-autoimmune-disease/