Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Adalimumab therapeutic drug monitoring (TDM) using established trough concentrations is not standard practice in JIA patients, unlike IBD. No specific guidelines outline a therapeutic trough level range or clinical indications for monitoring. The aim of this study was to evaluate our institution’s current practice of ordering and utilizing adalimumab trough levels for JIA patients.
Methods: Asingle-center, retrospective cohort study was completed using an electronic medical system. Non-systemic JIApatients on adalimumab with a minimum of one adalimumab trough drawn at our institution between 1/1/2019 to1/1/2022were included. Patient data, adalimumab dose and frequency, adalimumab trough levels, anti-drug antibody (ADA) activity, and subsequent treatment decisions were collected. The primary objective was to describe the therapeutic outcomes of drug level assessment in patients with JIA. Secondary objectives included indications for trough level testing, incidence of positive ADA activity, and treatment plan changes. Descriptive statistics were performed.
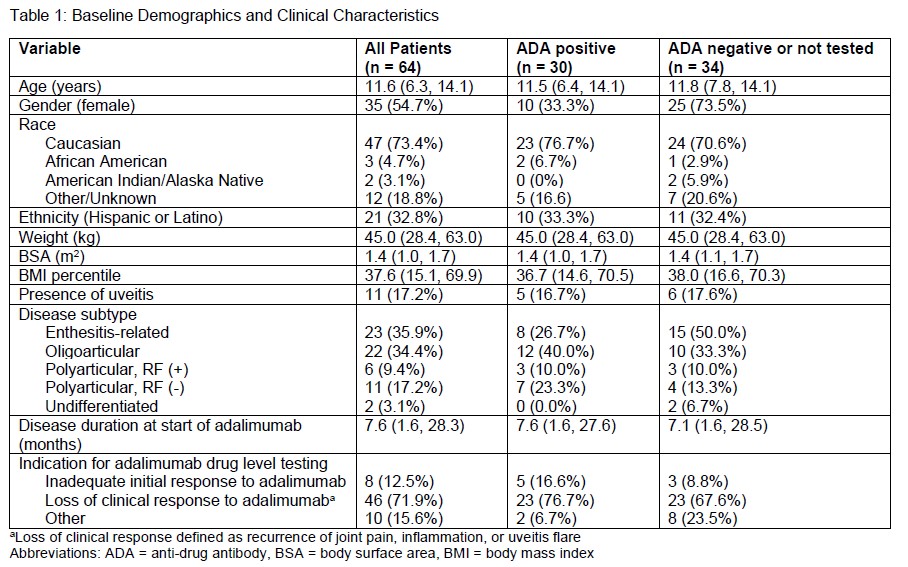
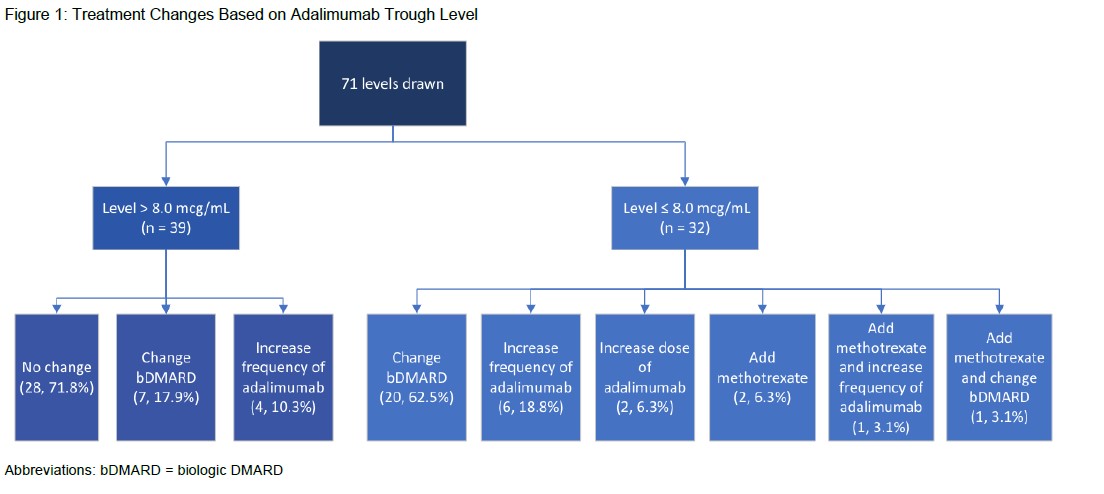
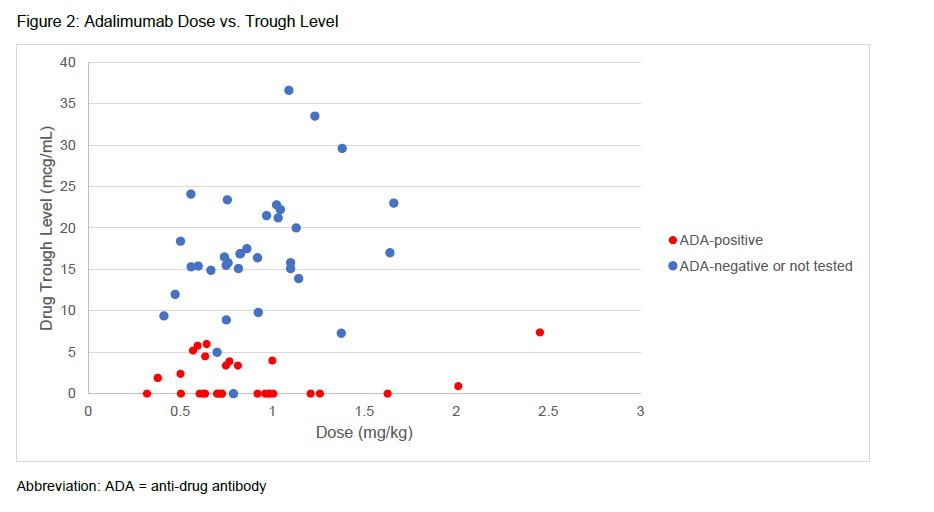
Results: A total of 64 patients with 71 adalimumab trough levels met study inclusion criteria of which 30 patients were ADA positive and 34 patients were ADA negative or not tested (Table 1).The median age was 11.6 years (6.3, 14.1), predominantly female (35, 54.7%) and Caucasian (47, 73.4%).Enthesitis-related arthritis (23, 35.9%) and oligoarticular JIA (22, 34.4%) were the most common subtypes. Common clinical indications for adalimumab drug level testing included loss of response (46, 71.9%) and inadequate initial response to adalimumab (8, 12.5%). Adalimumab troughs resulted at ≤ 8.0 mcg/mL in 32 (45.1%) measurements leading to ADA testing(Figure 1). Of patients tested for ADA, 30 patients were found to be positive. For patients with a level > 8.0 mcg/mL, the most common treatment decision was no change to therapy (28, 71.8%). Switching to an alternative biologic DMARD was the most common change (20, 62.5%) for ADA tested patients. Trough concentrations did not correlate with the dose of adalimumab that patients received (Figure 2).
Conclusion: No distinguishing patterns associated with ADA development were noted. Most trough concentrations collected were due to loss of clinical response to adalimumab. Assessing adalimumab trough concentrations impacted therapy changes, with switching to an alternative biologic DMARD as the most common practice for adalimumab levels ≤ 8.0 mcg/mL. Adalimumab doses did not correlate with trough concentration. Limitations include small sample size and chart review. Further studies are needed to identify the role of TDM in JIA patients.
To cite this abstract in AMA style:
Vallejos C, Cooper J, Pan I. Clinical Characteristics of Patients with Juvenile Idiopathic Arthritis Who Undergo Adalimumab Drug Level Testing and Anti-Drug Antibody Assessment [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-characteristics-of-patients-with-juvenile-idiopathic-arthritis-who-undergo-adalimumab-drug-level-testing-and-anti-drug-antibody-assessment/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-characteristics-of-patients-with-juvenile-idiopathic-arthritis-who-undergo-adalimumab-drug-level-testing-and-anti-drug-antibody-assessment/