Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab is the only non–tumor necrosis factor inhibitor biologic therapy approved for the treatment of ankylosing spondylitis (AS) in the United States. Patients with AS in clinical trials who receive biologics, including secukinumab, may not be representative of those treated in real-world clinical practice due to differences in patient characteristics, practice patterns, physician experience, and insurance coverage (ie, access). Few real-world studies have characterized patients with AS who initiate secukinumab. The Corrona PsA/SpA Registry collects data on a robust list of outcome measures, many of which overlap with those used in clinical trials. We aim to describe the characteristics of patients who initiated secukinumab and other biologics for the treatment of AS in the Corrona PsA/SpA Registry.
Methods: This study included patients aged ≥ 18 years with AS (and without concurrent PsA diagnosis) enrolled in the Corrona PsA/SpA Registry who initiated secukinumab or other biologics (adalimumab, etanercept, certolizumab pegol, infliximab, and golimumab) between April 2017 and December 2018. Patient demographics, clinical characteristics, treatment profiles, disease activity, quality of life, and work productivity were assessed at the time of biologic initiation (baseline) and compared between patients who initiated secukinumab and other biologics using t tests or Wilcoxon rank-sum tests for continuous variables and χ2 or Fisher exact tests for categorical variables.
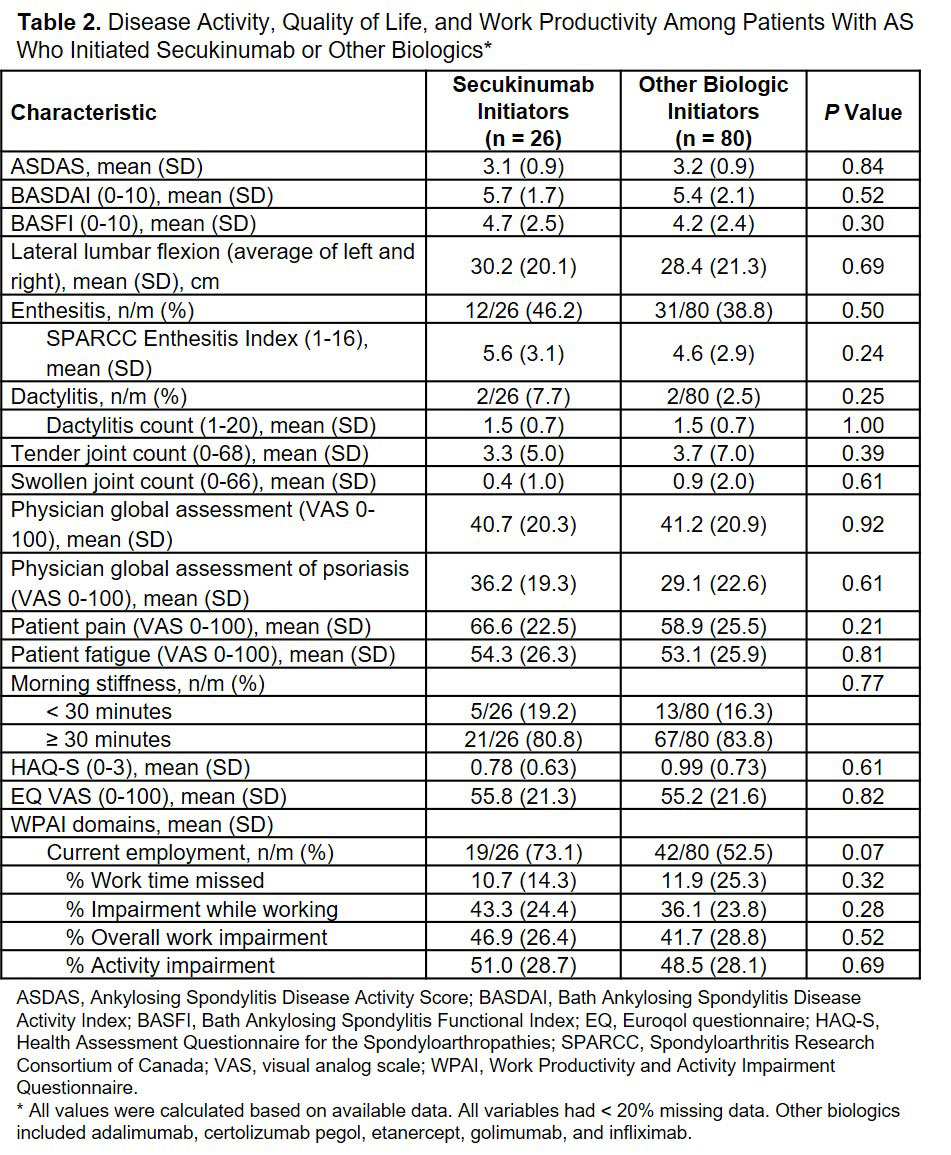
Results: Of the 106 patients with AS who initiated a biologic, 26 (24.5%) initiated secukinumab and 80 (75.5%) initiated other biologics. Secukinumab initiators were similar to patients who initiated other biologics in terms of demographics and clinical characteristics except that a higher proportion of secukinumab initiators had a history of prior biologic use (69.2% vs 45.0%; P = 0.03) and were more likely to have used a higher number of prior biologics compared with other biologic initiators (P < 0.01; Table 1). No significant differences were observed between patients who initiated secukinumab and other biologics in terms of measures of disease activity, quality of life, and work productivity (Table 2).
Conclusion:
In this real-world study of US patients with AS, secukinumab initiators had similar demographics, clinical outcomes, disease burden and patient reported outcomes compared with other biologic initiators, with the exception of higher prior biologic use.
To cite this abstract in AMA style:
Ogdie A, Liu M, Rebello S, Glynn M, Dube B, McLean R, Yi E, Park Y, Mease P. Clinical Characteristics and Treatment Profiles of Patients with Ankylosing Spondylitis Who Initiated Secukinumab and Other Biologics: Results from the Corrona Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinical-characteristics-and-treatment-profiles-of-patients-with-ankylosing-spondylitis-who-initiated-secukinumab-and-other-biologics-results-from-the-corrona-psoriatic-arthritis-spondyloarthritis-p/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-characteristics-and-treatment-profiles-of-patients-with-ankylosing-spondylitis-who-initiated-secukinumab-and-other-biologics-results-from-the-corrona-psoriatic-arthritis-spondyloarthritis-p/