Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Individuals with psoriasis or psoriatic arthritis (PsO/PsA) have an elevated risk of
major adverse cardiac events (MACE), which include congestive heart failure (CHF), myocardial
infarction (MI), and cerebrovascular accident (CVA). Biologic disease modifying antirheumatic
drugs (bDMARDs) may reduce cardiovascular risk through inflammation control and reduced
steroid exposure. Whether MACE risk differs by bDMARD class for individuals with psoriatic
disease is unknown.
Methods: We used data from the US-based TriNetX electronic health records database.
Patients were included if they had PsO/PsA and were new bDMARD users, including tumor
necrosis factor alpha inhibitors (TNFi), interleukin-17A inhibitors (IL17i), interleukin-23 inhibitors
(IL-23i), or interleukin-12/23 inhibitors (IL-12/23i). The time-dependent risk for MACE was
calculated using weighted multinomial Cox proportional hazards ratios with TNF exposure as
the referent. Subset analyses were performed to evaluate components of the primary outcome
measure and patients with or without baseline cardiovascular disease. A negative control
outcome (injury/trauma) was also evaluated.
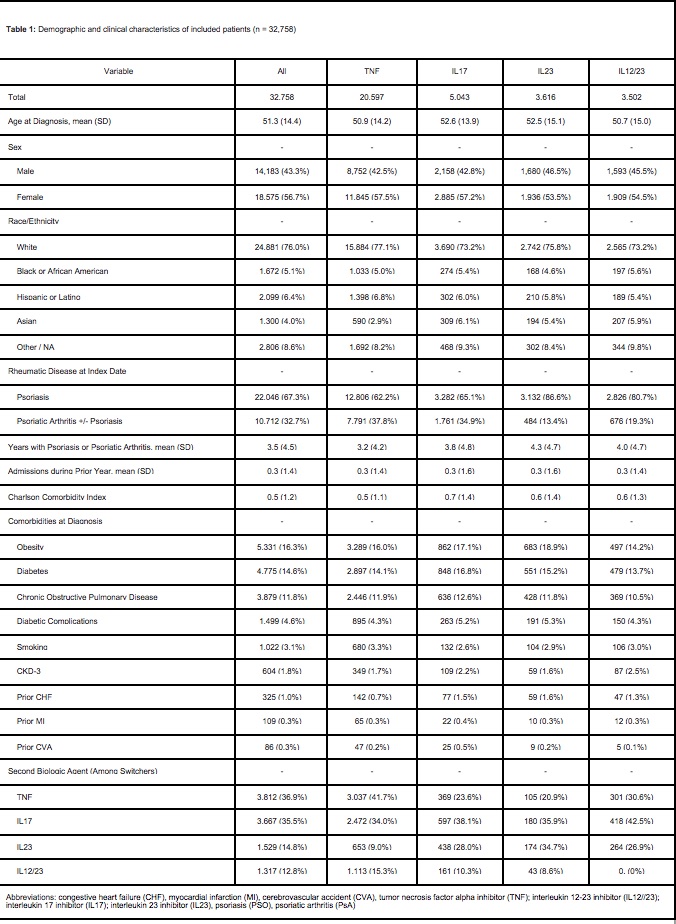
Results: We identified 32,758 patients who initiated biological therapies for PsO/PsA. Patients
had PsO/PsA for a mean of 3.5 years (SD 4.5) prior to starting a biologic agent, the most
common of which was a TNFi (20,597/32,758, 62.9%) followed by IL-17i (5,043/32,758, 15.4%),
IL-23i (3,502/32,758, 10.7%), and IL12/23i (3,502/32,758, 10.7%). In a weighted multinomial
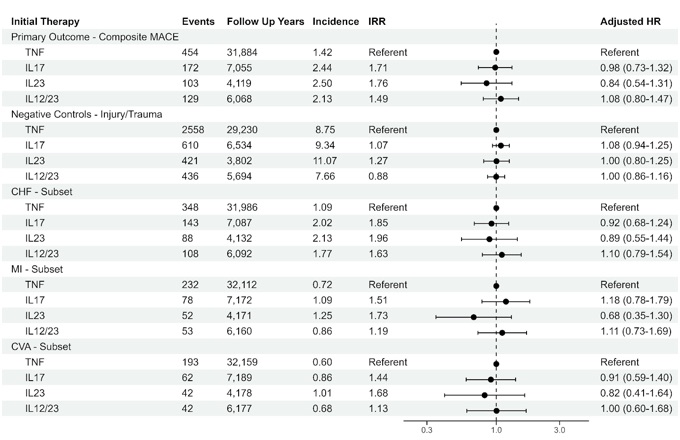
cox proportional hazards regression, the adjusted risk of MACE was similar for IL-17A inhibitors
(aHR 0.98, 95% CI 0.73-1.32), IL-23 inhibitors (aHR 0.84, 95% CI 0.54-1.31), and IL-12/23
inhibitors (aHR 1.08, 95% CI 0.80-1.47) as compared to TNF inhibitors. The results of subset
analyses supported the primary analysis. Negative control outcomes (injury/trauma) suggested
adequate control of time related biases and confounding.
Conclusion: Despite the differences in efficacy and safety profiles of bDMARD classes for
individuals with PsA/PsO, this real world analyses did not observe significant differences in
MACE risk with respect to biologic choice. Risk of MACE should not be a determining factor
when choosing a biologic agent for patients with psoriatic arthritis and psoriasis.
To cite this abstract in AMA style:
Gill B, Geiger J, Liew J, Putman M, Singla S. Choice of Biologic Immunotherapy for Psoriasis or Psoriatic Arthritis Not Associated with Risk of Major Adverse Cardiac Events [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/choice-of-biologic-immunotherapy-for-psoriasis-or-psoriatic-arthritis-not-associated-with-risk-of-major-adverse-cardiac-events/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/choice-of-biologic-immunotherapy-for-psoriasis-or-psoriatic-arthritis-not-associated-with-risk-of-major-adverse-cardiac-events/