Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: New onset inflammatory arthritis (IA) is reported in 6% of patients who receive immune checkpoint inhibitors (ICI). We previously developed an administrative claims-based algorithm to identify patients with ICI-IA.1 In this study, we compare ICI-IA cases at a single center identified in administrative claims and those identified via manual medical record review.
Methods: Using an Alteryx electronic health record (EHR) screening tool, we identified patients seen at Hospital for Special Surgery (HSS) who received ICI therapy between 2016-2025. Chart adjudication was performed independently by a team of three physicians to determine whether patients had ICI-IA. We defined ICI-IA as a condition developing within 1 year of receiving ICI, in patients without a prior diagnosis of inflammatory arthritis or PMR. The diagnosis was verified by 1. MD documentation in the EHR; or 2. treatment with steroids or DMARDs for joint symptoms; or 3. imaging or joint histopathology demonstrating joint inflammation. Patients with ICI-IA treated at HSS were also identified by applying our administrative claims algorithm to the de-identified Komodo Healthmap dataset which includes patients on commercial insurance, managed and fee for service Medicare.
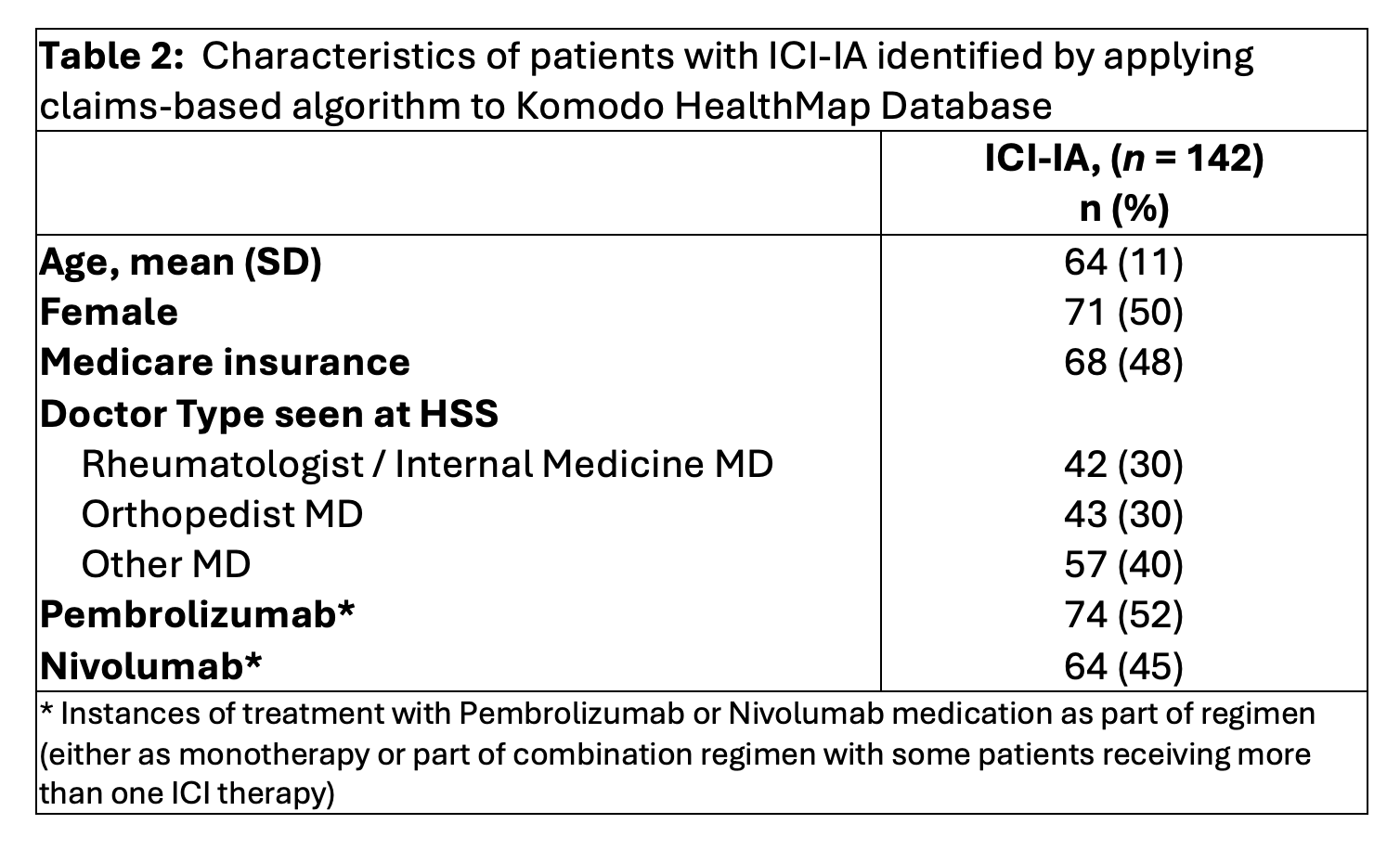
Results: ICI-treated patients seen at HSS (n=500) were identified and a random sample of 119 of these patients’ charts were reviewed and extracted. Of these, adjudicators recognized 53/119 (45%) as having ICI-IA (44 ICI-arthritis, 2 ICI-arthralgia, 7 ICI-PMR) and 66/119 (55%) as not having ICI-IA. Patients with ICI-IA had a mean (SD) age 62 (19) years, 52 (98%) were seen by a rheumatologist, 51(96%) were enrolled in an HSS irAE registry, and 46 (87%) received oral steroids for management of joint symptoms (Table 1). Of the patients who were seen at HSS for joint-related symptoms but did not have ICI-IA, 34 (51%) of patients had OA or other non-inflammatory joint pain (such as bursitis or tendonitis) leading to presentation and others had pre-existing inflammatory arthritis. The characteristics of patients identified as having ICI-IA who were treated at HSS in the unidentified Komodo dataset is shown in Table 2. In this cohort of 142 ICI-IA patients, average age (SD) was 64 (11), 71 (50%) were female, 68 (48%) were insured with Medicare, and 42 (30%) were seen by a rheumatologist or internal medicine provider.
Conclusion: Demographic characteristics and insurance of patients with ICI-IA identified through chart review and through query of the deidentified Komodo dataset were similar but fewer in Komodo were seen by a rheumatologist. In a future study using tokenization and linkage of the Komodo claims to cases identified at HSS, we will determine the positive predictive value of the ICI-IA algorithm.1Bass AR et al. Rheumatology (Oxford). 2025;64(4):1637-1642
To cite this abstract in AMA style:
Barasch J, Ghosh N, Jannat-Khah D, Ge K, Curtis J, Bass A. Checkpoint Inhibitor Inflammatory Arthritis: Single Center Case Identification and Chart Validation. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/checkpoint-inhibitor-inflammatory-arthritis-single-center-case-identification-and-chart-validation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/checkpoint-inhibitor-inflammatory-arthritis-single-center-case-identification-and-chart-validation/

.jpg)