Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: To better understand how US rheumatologists utilize biologics in the management of SLE, especially among those with cutaneous manifestations. No medication is currently FDA-approved specifically for cutaneous lupus erythematosus (CLE). Belimumab is approved for moderate-to-severe SLE and for LN. Anifrolumab, although FDA-approved for moderate-to-severe SLE, is undergoing Phase III clinical trials to evaluate its effectiveness for CLE and LN.
Methods: 1,036 moderate-to-severe adult SLE patient records were collected in collaboration with 176 US rheumatologists via an online survey from March to April 2024. These patients had to be treated with at least one prescription agent. This retrospective analysis focuses on the subset of 496 SLE patients treated with a biologic (belimumab, anifrolumab, or rituximab) at their most recent visit with their rheumatologist.
Results: Study results reveal that while belimumab is the most prescribed biologic in SLE, rheumatologists often employ anifrolumab in patients with cutaneous manifestations.
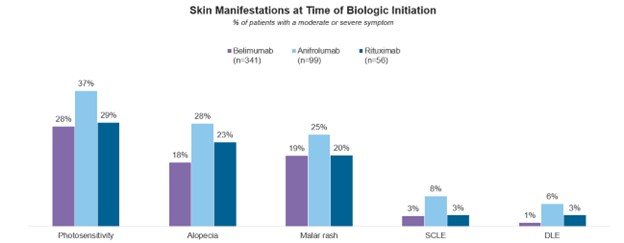
Nearly half (48%) of US moderate-to-severe SLE patients were prescribed a biologic at the most recent visit with their rheumatologist, with belimumab being the most prescribed (33%), followed by anifrolumab at 10% and rituximab at 5%. At treatment initiation, patients most commonly experienced pain/stiffness and fatigue; however, anifrolumab patients were more likely than belimumab and rituximab patients to present with skin manifestations including malar rash, photosensitivity, alopecia, discoid lupus erythematosus (DLE), and subacute cutaneous lupus erythematosus (SCLE).
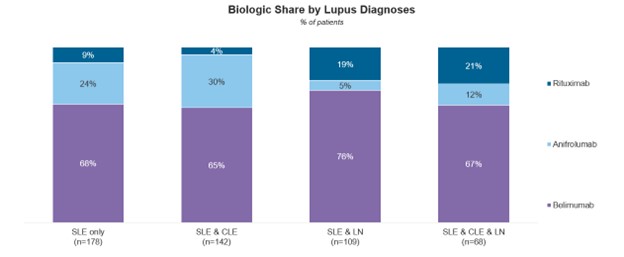
Among the audited patient population, 38% have been diagnosed with SLE only, 28% with both SLE and CLE but not LN, 22% with both SLE and lupus nephritis (LN) but not cutaneous lupus erythematosus (CLE), and 12% with all three conditions: SLE, CLE, and LN. While belimumab is the most prescribed biologic regardless of the combination of lupus diagnoses, rheumatologists prescribe anifrolumab the most in the patients with a CLE diagnosis, indicating a tailored approach based on specific disease manifestations.
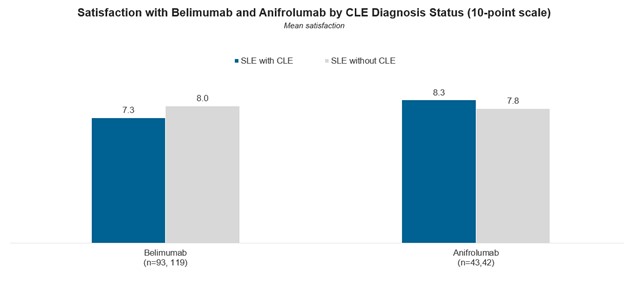
At a patient level, the analysis also reveals higher rheumatologist satisfaction with anifrolumab for patients diagnosed with CLE, while belimumab has higher satisfaction ratings among patients without CLE.
Conclusion: These findings highlight the individualized treatment strategies being employed in managing SLE, particularly in addressing the diverse manifestations of the disease in order to optimize patient outcomes. Further studies are warranted to establish the long-term efficacy and safety of anifrolumab in the treatment of CLE patients.
To cite this abstract in AMA style:
Yarnall M, Rex R, May S. Chart Audit of over 1,000 SLE Patients Reveals Biologic Treatment Choice Driven by Disease Manifestations [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/chart-audit-of-over-1000-sle-patients-reveals-biologic-treatment-choice-driven-by-disease-manifestations/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/chart-audit-of-over-1000-sle-patients-reveals-biologic-treatment-choice-driven-by-disease-manifestations/