Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Patients (pts) with mild to moderate psoriasis (PsO) and musculoskeletal disease burden may be diagnosed with PsA upon referral to a rheumatologist. Early intervention in pts with PsA may result in improved joint, skin, and quality-of-life (QoL) outcomes. This analysis of CorEvitas’ Psoriasis Registry sought to identify characteristics and musculoskeletal disease burden among pts naive to systemic treatment with mild to moderate psoriasis based on evidence of potential comorbid PsA.
Methods: The prospective, observational CorEvitas’ Psoriasis Registry enrolls adults with dermatologist-diagnosed PsO who initiated or switched to an eligible systemic treatment at enrollment or within 12 months before enrollment. Pts with mild to moderate PsO (Investigator Global Assessment: 2 or 3) initiating first systemic treatment at enrollment (April 2015‒June 2020) were grouped by musculoskeletal disease burden. This descriptive analysis compares pts with no evidence of PsA (no dermatologist-reported PsA and negative Psoriasis Epidemiology Screening Tool [PEST] screen [< 3]) to pts with dermatologist-reported PsA and to pts without dermatologist-reported PsA but positive PEST (≥3). Standardized differences were calculated (potentially meaningful difference: d > 0.1; moderate difference: d > 0.5).
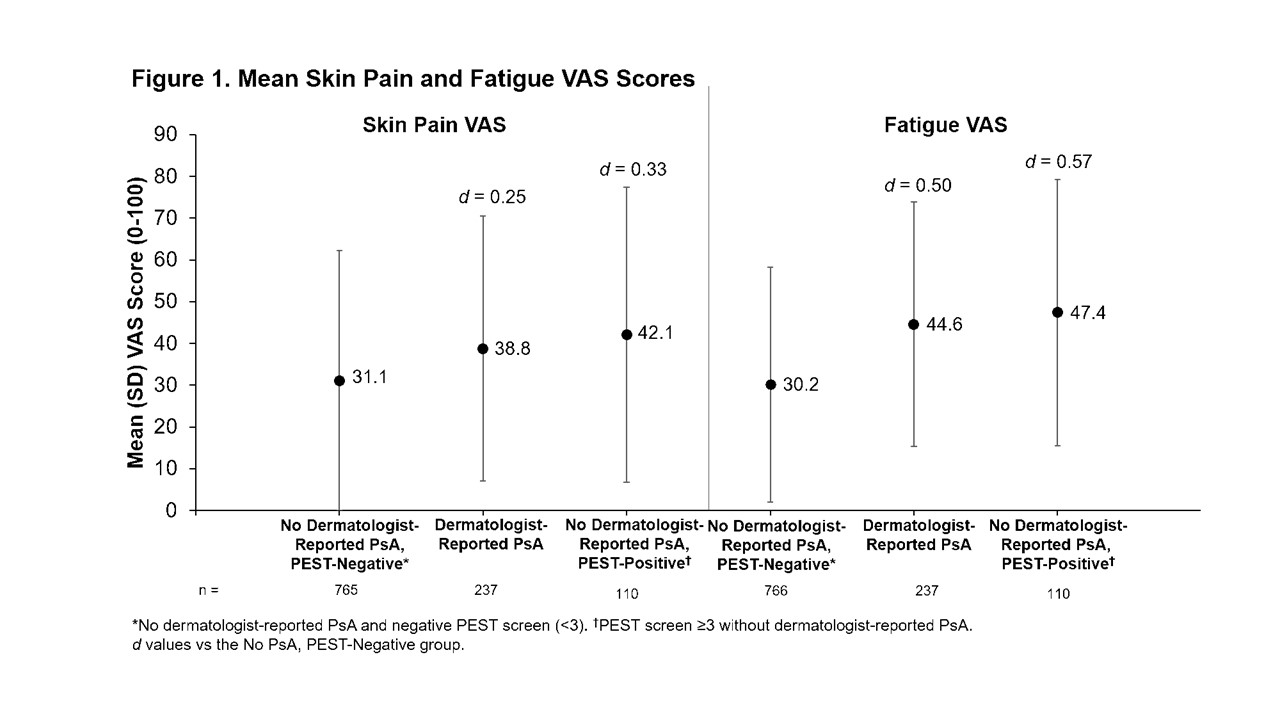
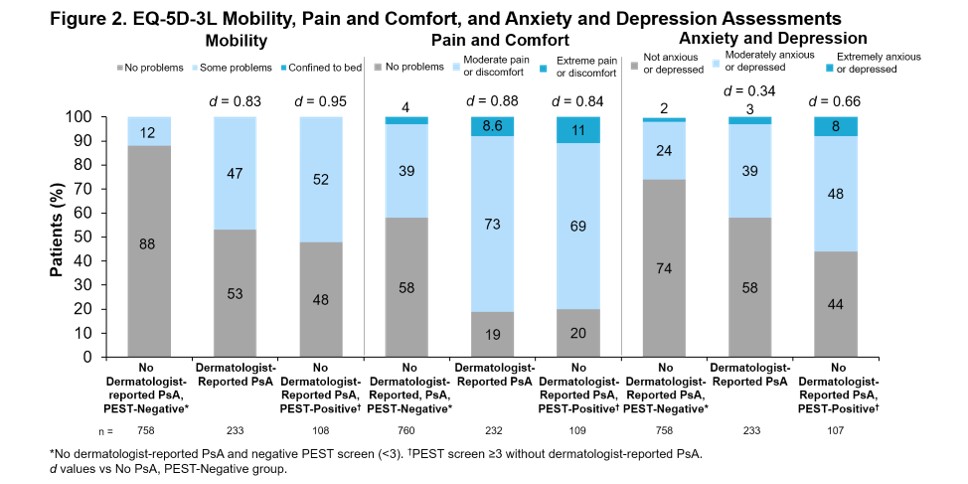
Results: Of the 1,113 pts enrolled, 766 (68.8%) had no evidence of PsA, 237 (21.3%) had dermatologist-reported PsA, and 110 (9.9%) had a positive PEST screen (PEST ≥3) without dermatologist-reported PsA. Concordance between dermatologist-reported PsA and positive PEST screen was 80%: 112 pts (10%) had dermatologist-reported PsA and negative PEST screen and 110 pts (10%) had positive PEST screen without dermatologist-reported PsA. For all groups, mean PsO duration was >9 years. For pts with dermatologist-reported PsA, mean PsA duration was 4 years (Table). Comorbidity histories were often higher in pts with dermatologist-reported PsA or positive PEST screen without dermatologist-reported PsA vs pts with no evidence of PsA (Table). Current employment was less prevalent among pts with dermatologist-reported PsA or positive PEST screen without dermatologist-reported PsA vs pts with no evidence of PsA (Table). Mean skin pain visual analog scale (VAS) and fatigue VAS scores were higher for pts with dermatologist-reported PsA or positive PEST screen without dermatologist-reported PsA vs no evidence of PsA (Figure 1). Also, EuroQol-5 Dimension 3 Level assessments indicated that pts with dermatologist-reported PsA or positive PEST screen without dermatologist-reported PsA had greater problems with mobility, pain and comfort, and anxiety and depression compared with pts with no evidence of PsA (Figure 2).
Conclusion: Pts naive to systemic treatment who have mild to moderate PsO and either PsA or musculoskeletal disease suggestive of comorbid PsA had greater comorbidity burden, symptom burden, and QoL impairment compared with pts with no evidence of PsA in this analysis from CorEvitas’ Psoriasis Registry.
To cite this abstract in AMA style:
Ogdie-Beatty A, Strober B, Lebwohl M, Cronin A, Lin T, Kang H, Middaugh N, O’Brien J, Jardon S, Richter S, Klyachkin Y, Mease P. Characterizing Musculoskeletal Disease Burden in Mild to Moderate Psoriasis Patients Suggestive of Comorbid PsA: Analysis of CorEvitas’ Psoriasis Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/characterizing-musculoskeletal-disease-burden-in-mild-to-moderate-psoriasis-patients-suggestive-of-comorbid-psa-analysis-of-corevitas-psoriasis-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterizing-musculoskeletal-disease-burden-in-mild-to-moderate-psoriasis-patients-suggestive-of-comorbid-psa-analysis-of-corevitas-psoriasis-registry/