Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Macrophage activation syndrome (MAS), also known as secondary hemophagocytic lymphohistiocytosis (HLH), is a life threatening condition that commonly presents with unremitting fever and shock like multi-organ dysfunction (MOD). Laboratory studies show pancytopenia, elevated liver enzymes, elevated ferritin, and hemophagocytosis. Familial forms of HLH result from homozygous defects in genes involved in perforin mediated cytolysis by NK cells and CD8 T cells. As many as 30-40% of MAS patient cohorts studied have heterozygous defects in the same HLH genes resulting in decreased cytolytic function, prolonged interaction with antigen presenting cells, and subsequent increased pro-inflammatory cytokines resulting in MOD. Since NK cell dysfunction is common in MAS, there are likely other genes that contribute to MAS via decreased cytolysis. Using gene sequencing, mutations in potentially novel HLH genes present in 2 or more MAS patients were explored.
Methods: Pediatric and adult patients with MAS at UAB were screened for genetic mutations, potentially contributing to MAS, via whole genome sequencing or a commercial immunodeficiency exomic genetic panel of 207 genes. Several patients were noted to have mutations in the guanine nucleotide exchange factor DOCK8 critical to NK cell function. DOCK8 mutations from this MAS cohort, or wild-type (WT) sequence controls, were introduced exogenously into human NK-92 NK cell lines by foamy virus (FV) transduction. Alternatively, the endogenous NK-92 DOCK8 genes were cut and repaired to express WT sequence or patient derived DOCK8 mutations by CRISPR/Cas9 technology. WT and mutant DOCK8 expressing NK-92 cells were incubated with K562 target cells and compared for cytolytic activity, degranulation (CD107a), and cytokine [interferon-g (IFNγ), tumor necrosis factor (TNF)] production by flow cytometry.
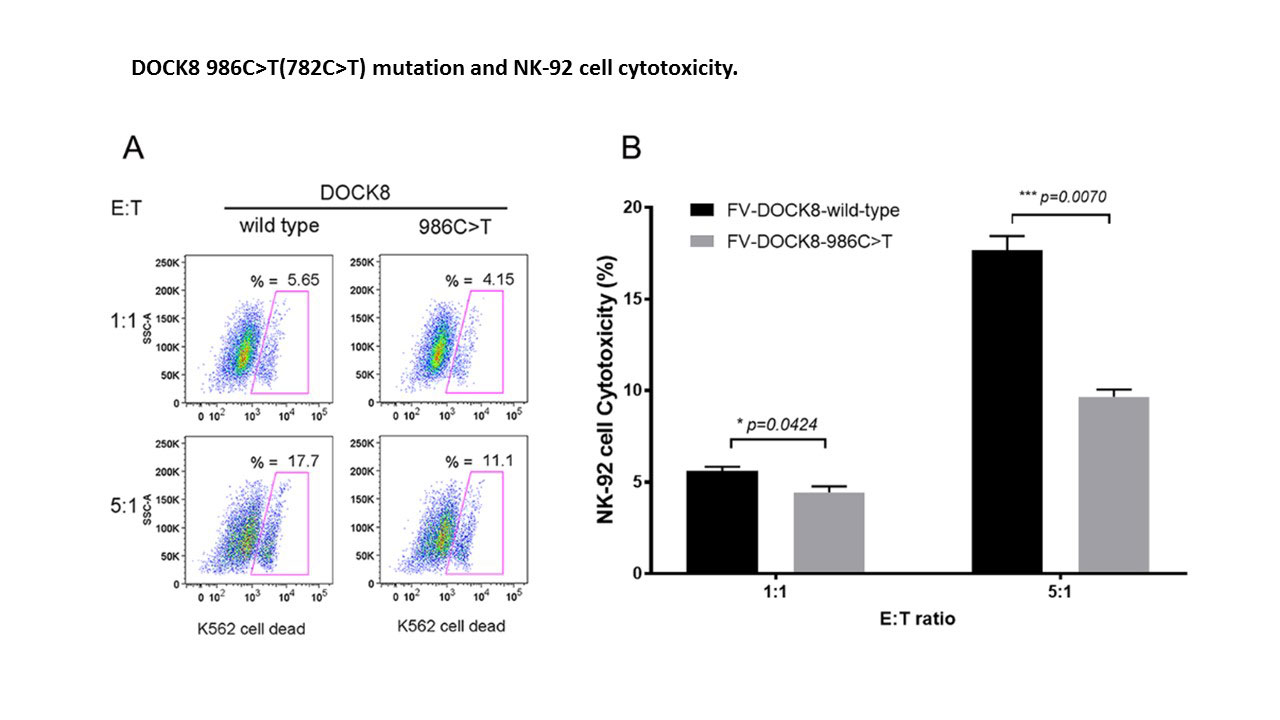
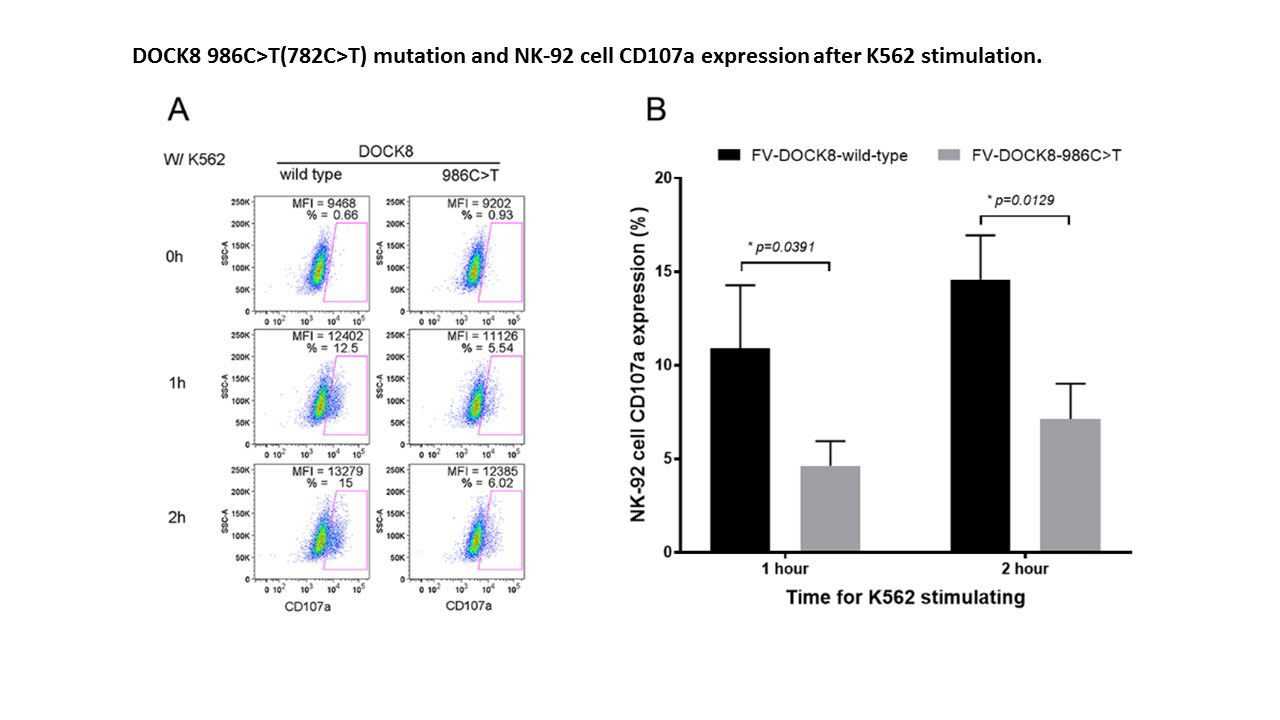
Results: Two MAS patients were identified with rare heterozygous DOCK8 mutations, and 2 others with MAS were noted to have the same DOCK8 polymorphism (c.187G >A, p.Asp63Asn) present in 12% of the population (Table). One of the rare mutations was missense (c.782C >T, p.Ala261Val – novel), and one was a splice acceptor variant (c.54-1G >T, 0.03%). The novel DOCK8 mutant consistently decreased NK cell lytic activity when introduced by either CRISPR/Cas9 (n=2) or FV (n=3, decreased by ~50% compared to WT, p=0.007) (Fig. 1). Similarly, the novel mutant decreased degranulation by >50% (n=3, p=0.0129) (Fig. 2). During the incubation of the NK-92 cells with K562 targets, NK cells expressing the novel DOCK8 mutant increased expression of IFNγ and TNF by >200% (p=0.0192 & p=0.0027, respectively). Prolonged interaction of the DOCK8 mutant NK-92 cells with K562 cells is currently being explored as a cause of increased cytokine production. Also, the DOCK8 splicing mutation is currently being tested functionally by “exon trapping” to explore a potential hypomorphic mutation.
Conclusion: Heterozygous mutations in DOCK8, a novel MAS associated gene, likely contribute to pathology through a partial dominant-negative or hypomorphic effect resulting in decreased cytolysis and increased pro-inflammatory cytokine production.
To cite this abstract in AMA style:
Zhang M, Cron R, Absher D, Crayne C, Atkinson P, Chatham W, Cron R. Characterization of DOCK8 as a Novel Gene Associated with Macrophage Activation Syndrome [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/characterization-of-dock8-as-a-novel-gene-associated-with-macrophage-activation-syndrome/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterization-of-dock8-as-a-novel-gene-associated-with-macrophage-activation-syndrome/