Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare inflammatory disorder characterized by asthma, eosinophilia, and small-to-medium size vessel vasculitis. In the Phase 3, double-blind, head-to-head MANDARA trial (NCT04157348) in patients with EGPA, benralizumab was non-inferior to mepolizumab in inducing remission, and the proportion of patients with relapse and time to first relapse were similar in the two treatment groups. This post hoc analysis aimed to describe the types of relapses that occurred in patients during the double-blind period.
Methods: Adults with relapsing or refractory EGPA on standard of care (oral glucocorticoids [OGC] ≥7.5 mg prednisone/prednisolone daily] ± other immunosuppressive therapy) received benralizumab 30 mg (n=70) or mepolizumab 300 mg (n=70) subcutaneously every 4 weeks for 52 weeks. At Week 4, the OGC dose could be tapered if disease was controlled (Birmingham Vasculitis Activity Score [BVAS] =0). Relapse was defined as the presence of active vasculitis (BVAS >0), active asthma, or active sinonasal disease leading to an increase in OGC doses to >4 mg/day, initiation or increase in immunosuppressive therapy, or hospitalization for worsening EGPA. In this post hoc analysis, each relapse was classified as asthma-related, sinonasal-related, or “other” (i.e. not asthma or sinonasal), or a combination, as follows: asthma relapse was based on wheeze on BVAS or increase in the 6-item Asthma Control Questionnaire [ACQ-6] score; sinonasal relapse was based on BVAS paranasal sinus involvement or bloody nasal discharge/crusts/ulcers/granulomata.
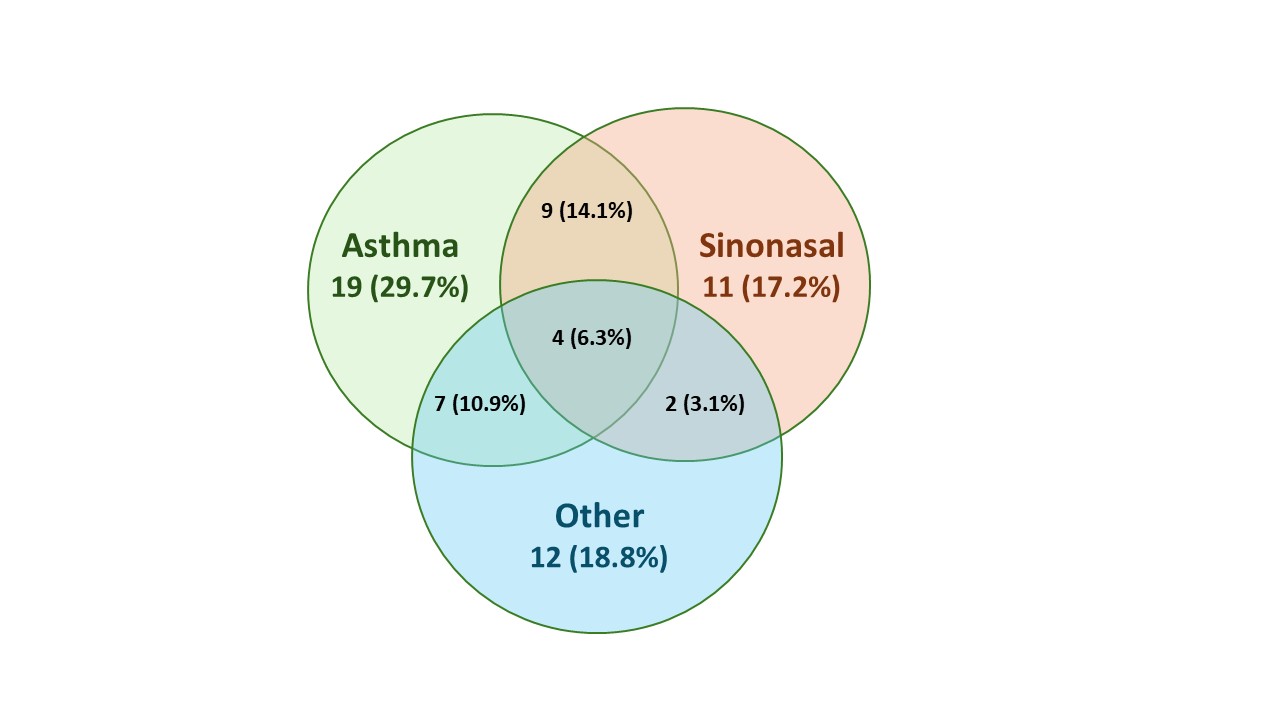
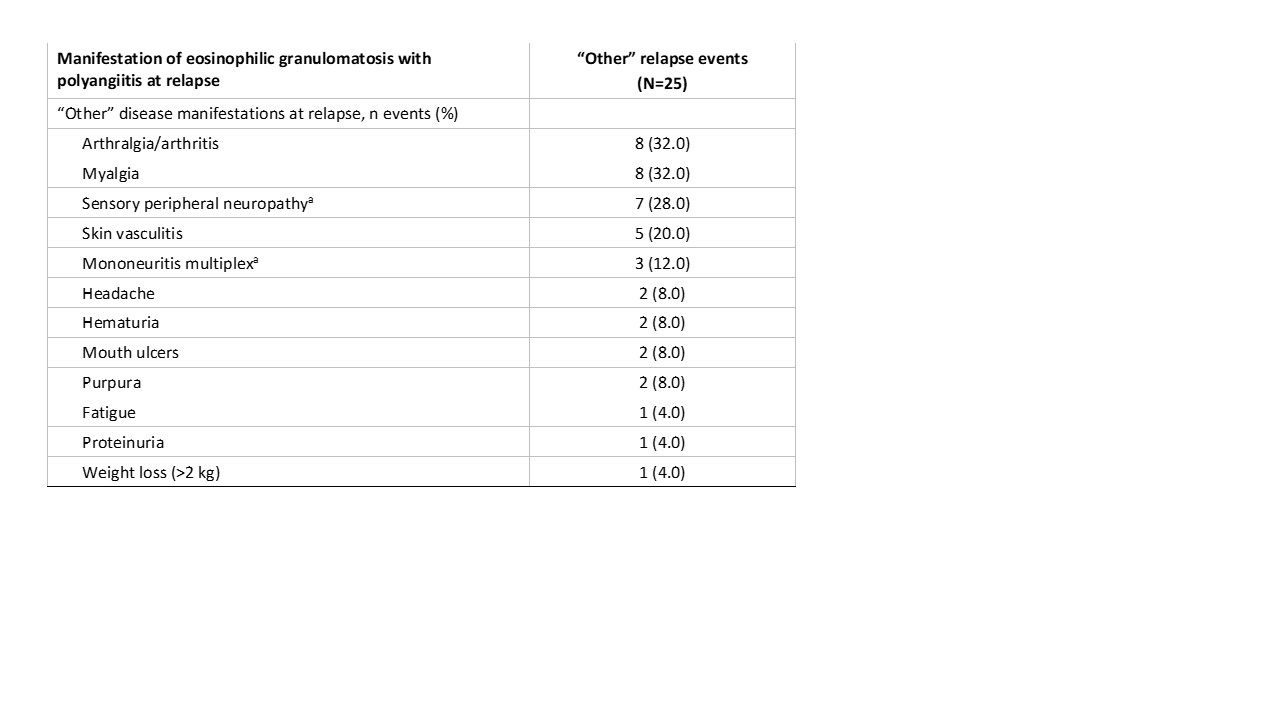
Results: 140 patients with EGPA were included in the analysis (benralizumab, n=70; mepolizumab, n=70). Mean (SD) age was 52.3 (14.10) years and 60.0% of patients were female. During the double-blind period, 64 relapses (any subtype) were reported in 42 (30.0%) patients (benralizumab: 34 relapses in 21 patients; mepolizumab: 30 relapses in 21 patients) (Figure). 52/64 (81.3%) relapses involved the airways with 39/64 (60.9%) relapses exclusively airway-related, including 19 (29.7%) asthma relapses, 11 (17.2%) sinonasal relapses, and 9 (14.1%) combined asthma and sinonasal relapses. 25/64 (39.1%) relapses involved a non-airway manifestation of EGPA of which 12/64 (18.8%) relapses were exclusively categorized as “other” (i.e. neither asthma or sinonasal). 22/64 relapses (34.4%) were combination relapses. The most common manifestations of disease associated with the 25 “other” relapses were arthralgia/arthritis (8 [32.0%]), myalgia (8 [32.0%]), sensory peripheral neuropathy (7 [28.0%]), and skin vasculitis (5 [20.0%]) (Table).
Conclusion: In this analysis of the MANDARA trial, the majority of relapses in patients with relapsing or refractory EGPA treated with either benralizumab or mepolizumab were airway-related (asthma and/or sinonasal), although non-airway manifestations of disease were also common during relapses.
Numbers denote the number of relapses by subtype out of a total of 64 relapses. Percentages are based on the total number of relapses. Each relapse was classified as asthma related, sinonasal related, “other”, or a combination, based on which relapse criteria were met.
Percentages are based on the number of relapse events in “other” category. Each relapse may be associated with more than one manifestation of disease.
aOne patient had a relapse that included both sensory peripheral neuropathy and mononeuritis multiplex.
To cite this abstract in AMA style:
Merkel P, Jayne D, Specks U, Pagnoux C, Nair P, Khalidi N, Bourdin A, Börjesson Sjö L, Necander S, Shavit A, Walton C, Wechsler M. Characteristics of Relapses in Patients with Eosinophilic Granulomatosis with Polyangiitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/characteristics-of-relapses-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-of-relapses-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis/