Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Janus kinase inhibitors (JAKinib) are effective in patients with rheumatoid arthritis (RA); however, some patients show an inadequate response to JAKinibs (JAKinib-IR). This study aimed to determine the clinical characteristics of JAKinib-IR patients with RA and identify suitable molecular-targeted drugs for such patients.
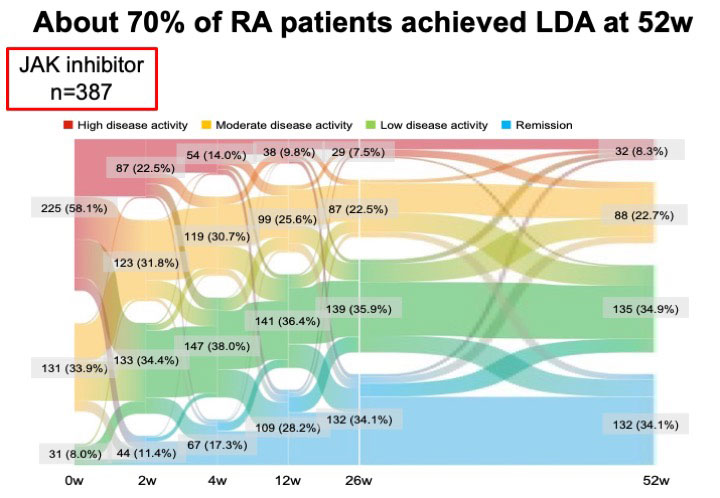
Methods: The subjects were 387 patients with RA and were recruited from the FIRST registry, a registry study of patients with RA receiving molecularly targeting therapies at multiple centers. The JAKinib (tofacitinib, 162 patients; baricitinib, 183; peficitinib, 8; upadacitinib, 30; and filgotinib, 4) were administered to the patients and the JAKinib-IR were defined by CDAI >10.0 at week52 despite treatments with JAKinibsm which were switched to a biologic DMARD or another JAK inhibitor. The efficacy and safety of switched molecular-targeted drugs were analyzed six months after switching treatment to identify suitable molecular-targeted drugs in JAKinib-IR patients.
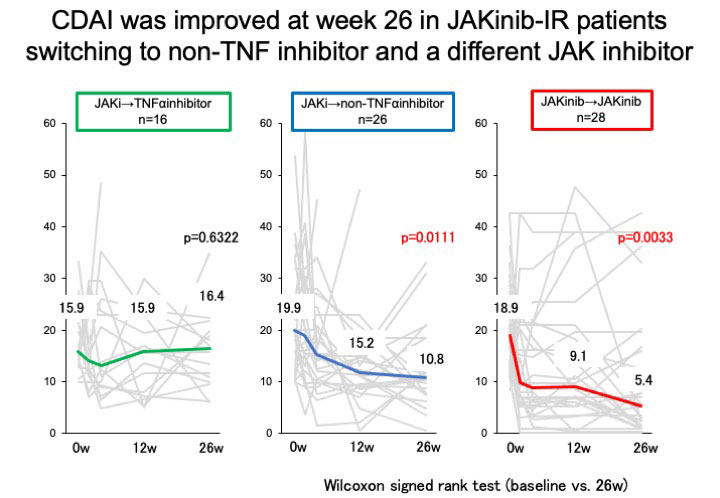
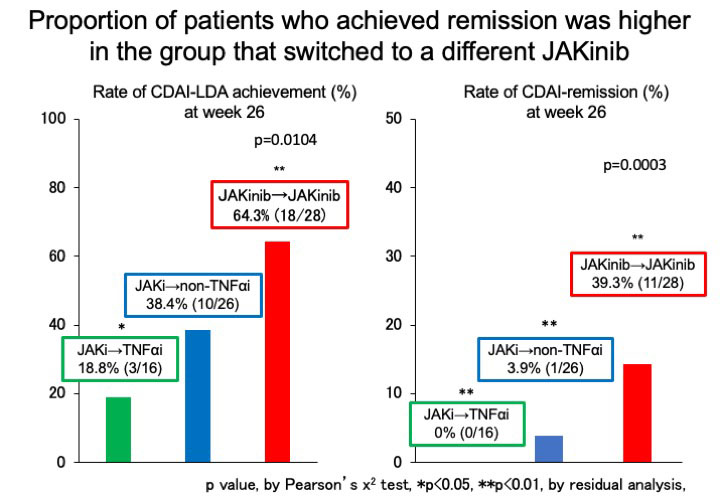
Results: Overall, 70 (18.1%) patients were JAKinib-IR one year after the introduction of JAKinibs. The factors associated with JAKinibs-IR were identified only the numbers of previous biologics use (Odds ratio (OR) 1.26, 95% confidence interval (CI) 1.08-1.47, p=0.0041) by multivariable logistic regression analysis.Out of 70 JAKinib-IR patients who switched to another molecular-targeted drugs (TNFα inhibitor [TNFi], 16; non-TNFi, 26 (IL-6 receptor inhibitor, 19; abatacept, 7); JAKinib, 27), 12 (17.1%) patients achieved CDAI remission. There was no significant difference in the patient characteristics at the time of switching treatment among the three groups (group1: patients who switched to TNFi, group2: patients switched to non-TNFi, group3: patients switched to different JAKinib). The retention rates over 26 weeks did not differ among the three groups (group1, 75.0% (12/16); group2, 80.8% (21/26); group3, 85.7% (24/27), p=0.5766)There was no significant difference in the rate of adverse events leading to discontinuation of molecular target drugs among three groups. JAKinib-IR patients switching to TNFi did not exhibit a significant decrease in clinical disease activity index (CDAI) at 26 weeks. CDAI was improved at week 26 in JAKinib-IR patients switching to non-TNF inhibitor and a different JAKinib. The proportion of patients who achieved CDAI-remission was significantly higher in the group that switched to a different JAKinib (39.3%) than in the other drug groups (TNFi, 0.0%; non-TNFi, 3.9%; p< 0.001). The factors associated with CDAI-remission in JAKinib-IR patients were identified only switching to a different JAKinib (OR 27.57, 95%CI 3.08-246.6, p=0.003) by logistic regression analysis.
Conclusion: Seventy of 387 RA patients (18.1%) were JAKinib-IR in the FIRST registry. Only a number of prior bDMARDs at baseline affected JAKinib-IR by multi-variable analysis. Switch to another JAKinib was significantly more effective than switch to TNF-inhibitors in patients with the first JAKinib-IR.
To cite this abstract in AMA style:
Miyazaki Y, Nakayamada S, Kubo S, Sonomoto K, Inoue Y, fukuyo s, Hanami K, Tanaka Y. Characteristics and Treatment-selection in Patients with Rheumatoid Arthritis and with Inadequate Response to Janus Kinase Inhibitors [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/characteristics-and-treatment-selection-in-patients-with-rheumatoid-arthritis-and-with-inadequate-response-to-janus-kinase-inhibitors/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-and-treatment-selection-in-patients-with-rheumatoid-arthritis-and-with-inadequate-response-to-janus-kinase-inhibitors/