Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a heterogeneous chronic inflammatory disease affecting the joints, skin, and other regions of the body. It impacts up to 36% of patients with psoriasis in the US, often substantially impairing quality of life.1,2 Biologic or targeted synthetic (b/ts) DMARDs, including interleukin (IL)-17, IL-12/23, tumor necrosis factor (TNF), and Janus kinase (JAK) inhibitors (i), have been effective in improving symptoms, but patients may experience suboptimal response or loss of response despite undergoing multiple lines of treatment.2,3 This study analyzed characteristics and treatment patterns of patients with PsA upon initiation of a third-line or higher (3L+) b/tsDMARD therapy. Understanding these patterns may offer insights into the benefits of managing PsA in its early stages with effective treatments.
Methods: This retrospective cohort study included patients who were ≥18 years of age, had a PsA diagnosis, and initiated a b/tsDMARD between January 2016–December 2023 as a 3L+ therapy at or after entry into the CorEvitas PsA/Spondyloarthritis Registry (baseline), a prospective, multicenter, observational US registry. Patient characteristics, treatment histories, and treatment patterns were assessed at baseline. Continuous variables were reported as means with standard deviation (SD) or medians with interquartile range (IQR), while categorical variables were described with counts and percentages.
Results: The CorEvitas registry included 7,044 initiations of b/tsDMARDs, with 2,550 (36.2%) being 3L+ initiations. This analysis included 1,210 patients initiating their first eligible 3L+ b/tsDMARD therapy (Figure 1). The mean (SD) age was 54.2 (12.3) years, 721 (59.7%) patients were female, and 1,105 (92.9%) patients were white. Two or more comorbidities were observed in 446 (36.9%) patients. The most common comorbidities were obesity, hypertension, and depression, reported in 734 (61.6%), 484 (40.0%), and 262 (21.7%) patients, respectively. Patients had a median (IQR) PsA duration of 7.0 (3.0, 12.0) years and symptom duration of 10.0 (5.0, 18.0) years. The median (IQR) time from first b/tsDMARD therapy to initiation of current therapy was 1.5 (0.7, 2.9) years. Most patients (813 [94.2%]) reported active disease as the reason for initiating a 3L+ therapy. Patients frequently used two prior bDMARDs (700 [57.9%]), while 1,102 (91.1%) were tsDMARDs naïve (Table 1). Concomitant conventional synthetic DMARDs were taken by 328 (27.1%) patients. Biologics most often used immediately prior to 3L+ therapy were TNFis (726 [68.5%]) or IL-17is (182 [17.2%]) (Figure 2). The most commonly initiated 3L+ b/tsDMARD class was IL-17i, used by 455 (37.6%) patients, followed by TNFis (338 [27.9%]), IL-23is (169 [14.0%]), and JAKis (148 [12.2%]).
Conclusion: These findings highlight the disease burden in this heavily pre-treated population, underscoring the need for early, more effective PsA treatments that provide a sustained response.
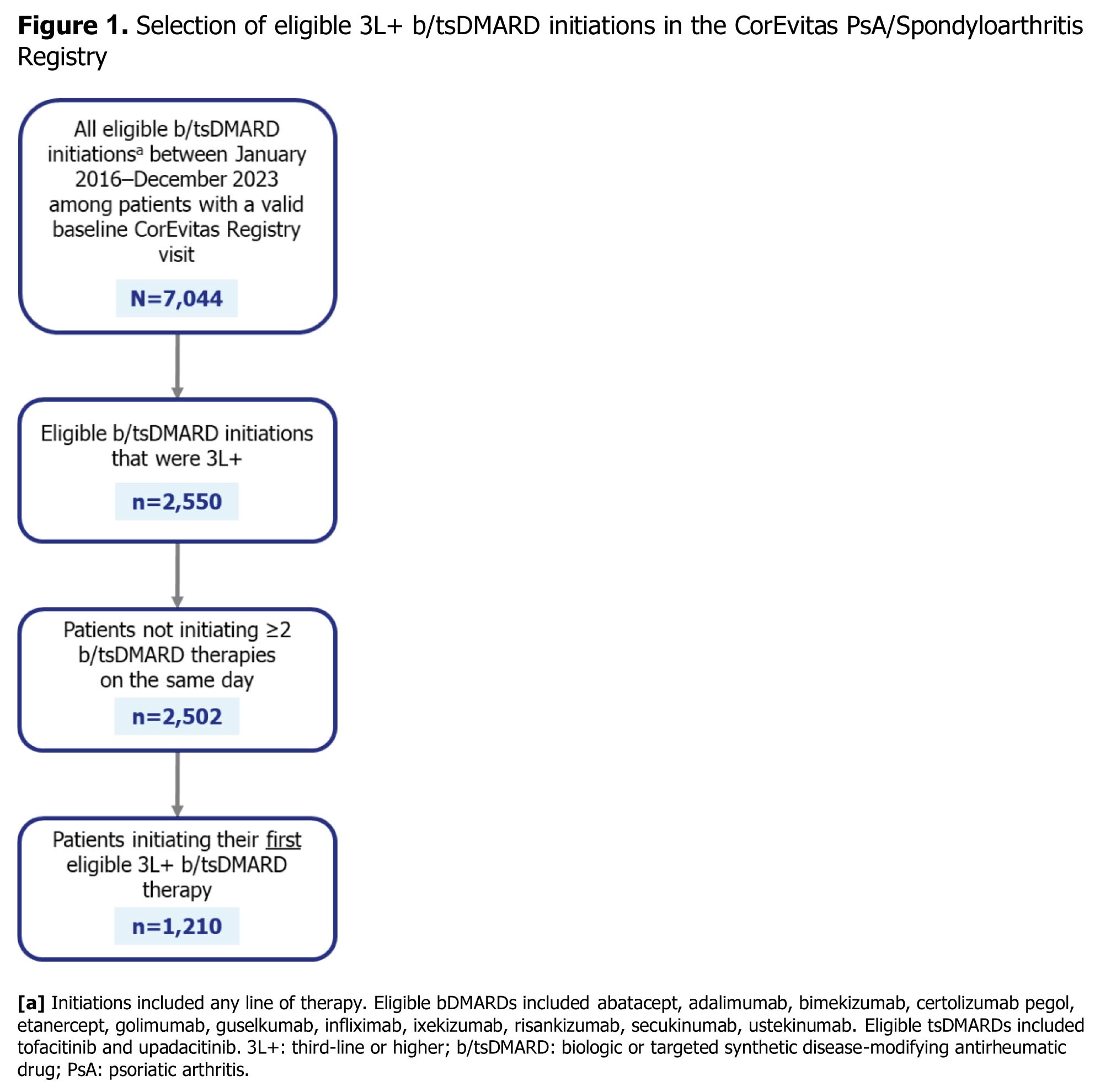 Figure 1. Selection of eligible 3L+ b/tsDMARD initiations in the CorEvitas PsA/Spondyloarthritis Registry
Figure 1. Selection of eligible 3L+ b/tsDMARD initiations in the CorEvitas PsA/Spondyloarthritis Registry
.jpg) Table 1. Baseline characteristics and disease activity measures of patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry
Table 1. Baseline characteristics and disease activity measures of patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry
.jpg) Figure 2. Current and immediate prior treatment patterns of patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry at baseline
Figure 2. Current and immediate prior treatment patterns of patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry at baseline
To cite this abstract in AMA style:
Mease P, Middaugh N, Muñoz Maldonado Y, Song C, Eliot M, Low R, Ogdie A. Characteristics and Treatment Patterns among Patients with Psoriatic Arthritis in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry Initiating a Third or Higher Line of Biologic or Targeted Disease-Modifying Antirheumatic Drug Therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/characteristics-and-treatment-patterns-among-patients-with-psoriatic-arthritis-in-the-corevitas-psoriatic-arthritis-spondyloarthritis-registry-initiating-a-third-or-higher-line-of-biologic-or-targeted/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-and-treatment-patterns-among-patients-with-psoriatic-arthritis-in-the-corevitas-psoriatic-arthritis-spondyloarthritis-registry-initiating-a-third-or-higher-line-of-biologic-or-targeted/
