Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The treatment armamentarium with immunosuppressive treatments (ISTs) for interstitial lung disease (ILD) in systemic sclerosis (SSc) has greatly expanded. It is to date unclear to which degree and its impact on ILD progression, which is of importance for the management of patients and clinical trials. Here, we assess treatment regimens and its impact on ILD progression and mortality in SSc-ILD.
Methods: SSc patients registered in the EUSTAR database with presence of ILD on imaging, available FVC%, DLCO% predicted and treatments at baseline and at 12 ± 3 months were included. ILD progression was defined as absolute FVC≥5% or DLCO≥10% decline. We segregated treatment regimens into four periods based on patients first assessment: 1) ≤ 2006; 2) 2007-2011; 3) 2012-2016; 4) ≥ 2017 and assessed clinical characteristics and type of IST. Next, we evaluated the impact of ISTs across the periods on ILD progression and a combined morbidity-mortality endpoint (progression and 3-year death). Lastly, we assessed the impact of ISTs on these endpoints in patients with positive anti-topoisomerase I (ATA) and short disease duration (≤60 months since first non-Raynaud’s symptom) in a subgroup analysis. Non-parametric tests were used for multiple group comparisons.
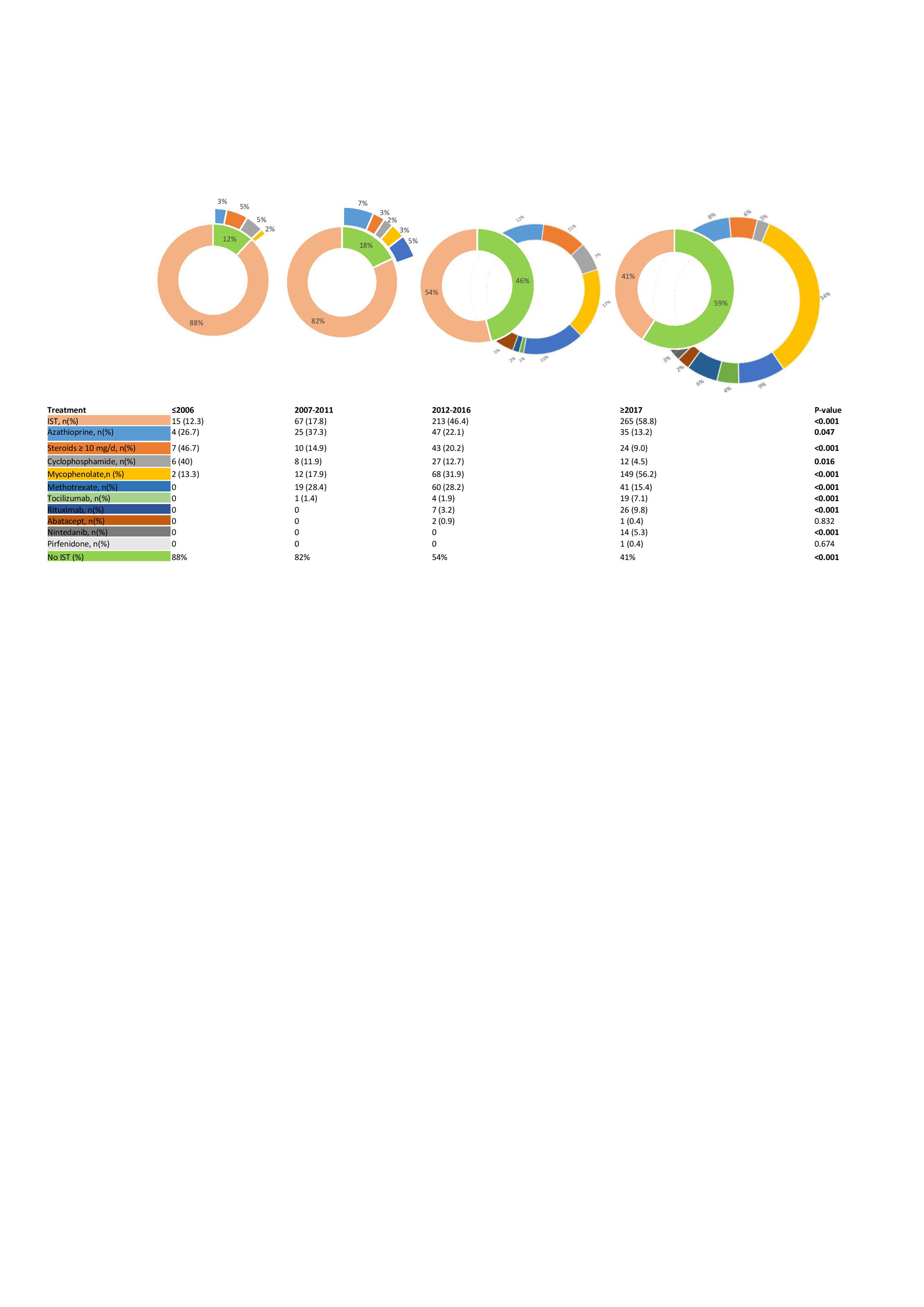
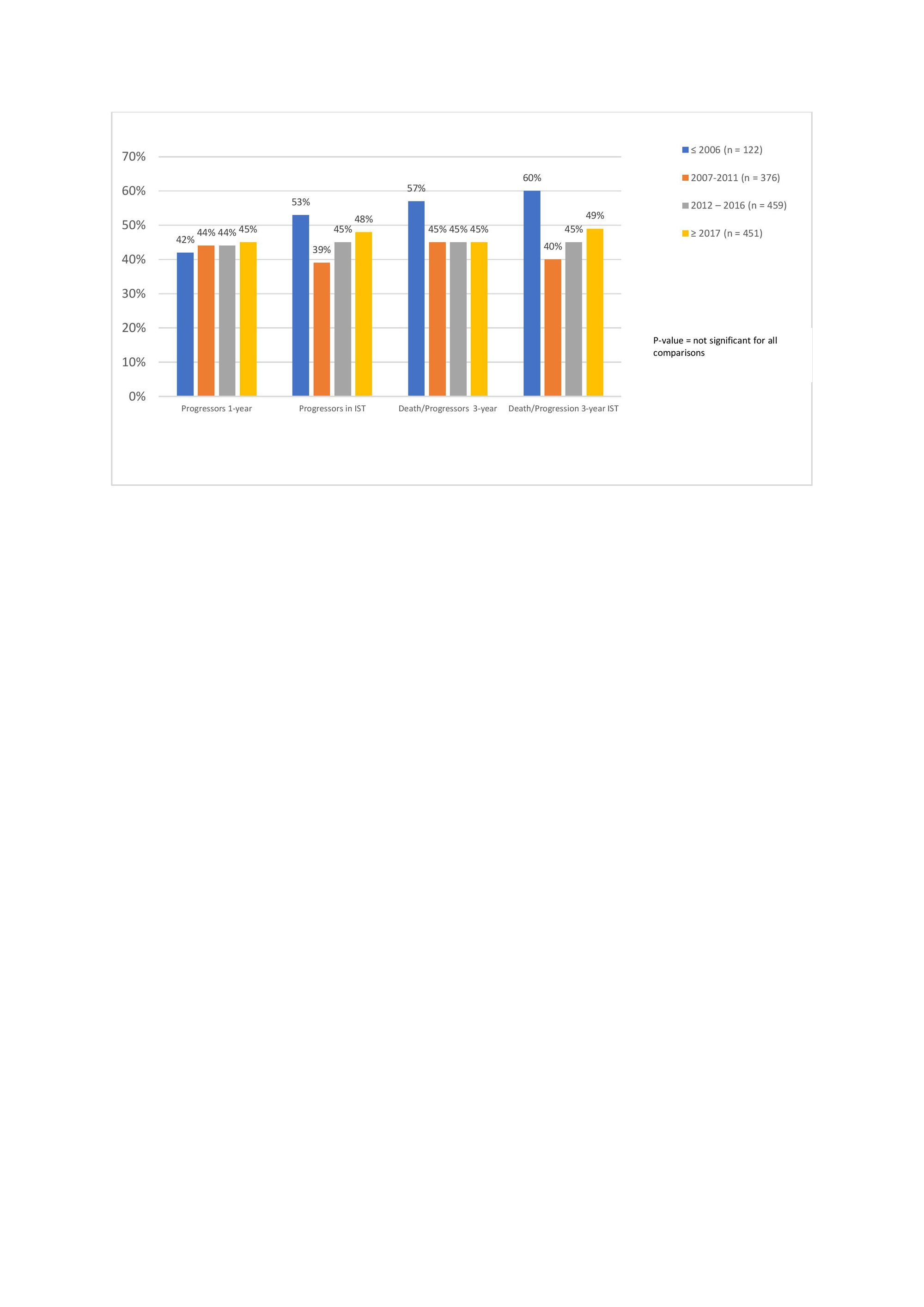
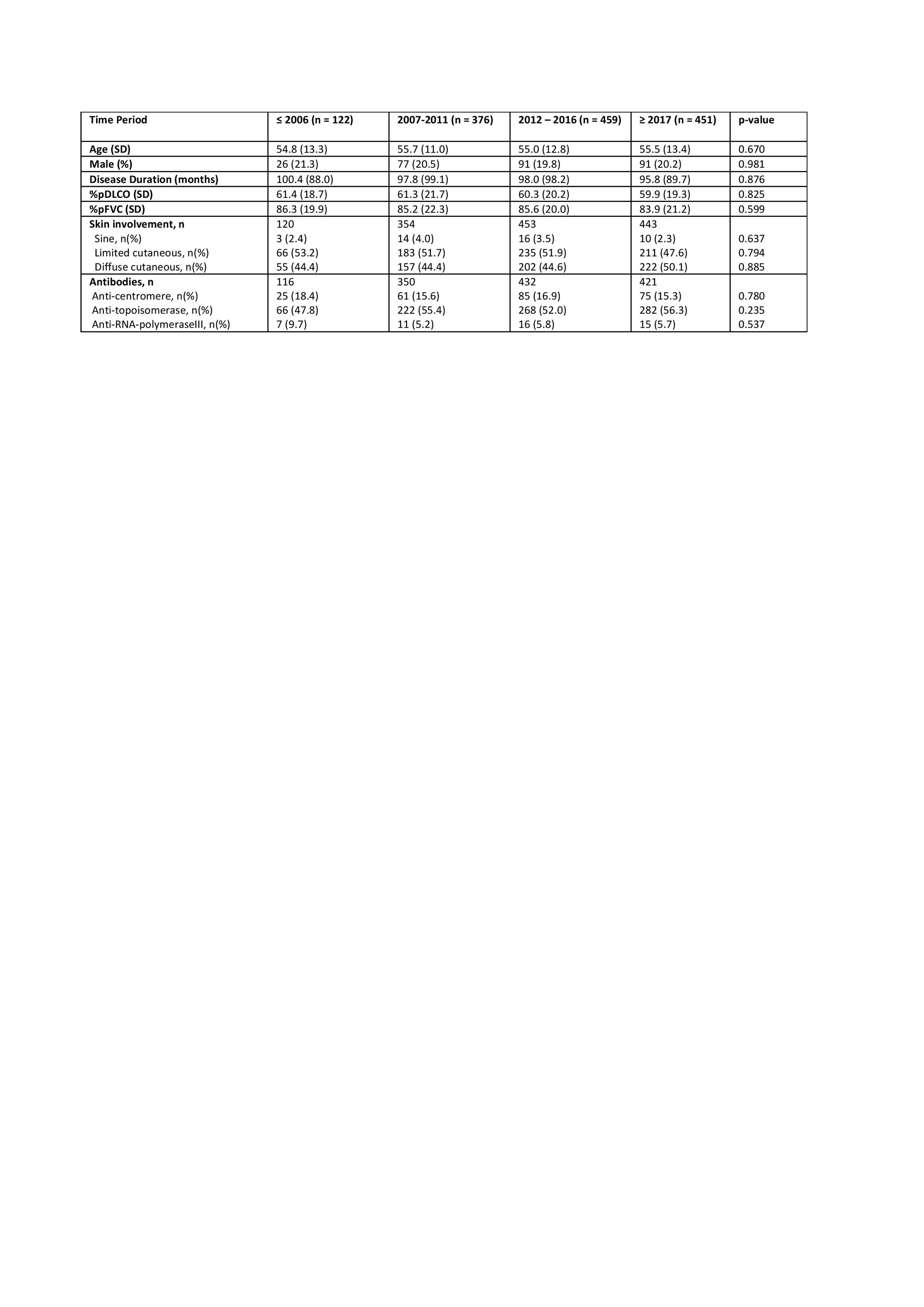
Results: We included 1408 SSc–ILD patients with 122 (8.7%) in period 1; 376 (26.7%) in period 2; 459 (32.6%) in period 3 and 451 (32.0%) in period 4. We did not identify major differences in clinical characteristics across the periods (Table 1); whereas the ISTs changed significantly from 12 % treated with IST in period 1 to 59% in period 4 (Figure 1). When assessing the impact of ISTs across the periods on our endpoints, we did not identify significant impact on ILD progression or the morbidity-mortality (Figure 2). In the subgroup analysis, enriching for progressive patients with short disease duration and ATA, we included 324 (23%) SSc-ILD patients across the 4 periods (4, 32, 120 and 168 in period 1-4). In this analysis, excluding those from period 1 due to low numbers, we identified significantly less ILD progression with 59.4%, 37.5% and 35.1%, respectively (p =0.037) and morbidity-mortality endpoint with 62.5%, 38.3% and 38.7%, respectively (p=0.036).
Conclusion: We show that treatment with immunosuppressives has increased significantly over the past decade, with currently 60% of SSc-ILD patients on IST. This is important knowledge for the design and inclusion of patients into clinical trials. We do not identify an impact on ILD progression in the total cohort but in an enriched population, showing the importance of treatment, specifically of patients at risk for progression.
To cite this abstract in AMA style:
Campochiaro C, Truchetet M, Vonk M, Cuomo G, Ananieva L, Hachulla E, Smith V, Gheorghiu A, Becvar R, Hunzelmann N, Furst D, Ortiz-Santamaria V, Carreira P, Del Galdo F, Matucci Cerinic m, Hoffmann-Vold A. Changes in Treatment Patterns and Their Influence on the Outcome of Systemic Sclerosis-interstitial Lung Disease (SSc-ILD) Patients: An EUSTAR Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/changes-in-treatment-patterns-and-their-influence-on-the-outcome-of-systemic-sclerosis-interstitial-lung-disease-ssc-ild-patients-an-eustar-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-treatment-patterns-and-their-influence-on-the-outcome-of-systemic-sclerosis-interstitial-lung-disease-ssc-ild-patients-an-eustar-analysis/