Session Information
Date: Tuesday, November 12, 2019
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In the double-blind, placebo-controlled 16-week pilot including 10 systemic sclerosis (SSc) patients with upper and lower gastrointestinal (GI) symptoms we found that fecal microbiota transplantation (FMT) with commercially-available anaerobic cultivated human intestinal microbiota (ACHIM) reduced lower GI symptoms. Here, we aimed to determine relative abundance of gut microbiota at baseline and changes after FMT.
Methods: Gastroduodenoscopy transfer of ACHIM or placebo was performed at weeks 0 and 2; fecal material was collected at week 0, 4 and 16. Relative abundance of total, immunoglobulin (Ig) A- and IgM-coated fecal bacteria was measured by 16s rRNA sequencing at week 0, 4 and 16. Relative abundance and taxonomic profiles were computed, alpha diversity and beta diversity were determined. Linear mixed models analysis were used to estimate changes in the microbiota composition and in IgA and IgM coating patterns over the entire follow-up period (i.e. baseline, 4 weeks, and 16 weeks).
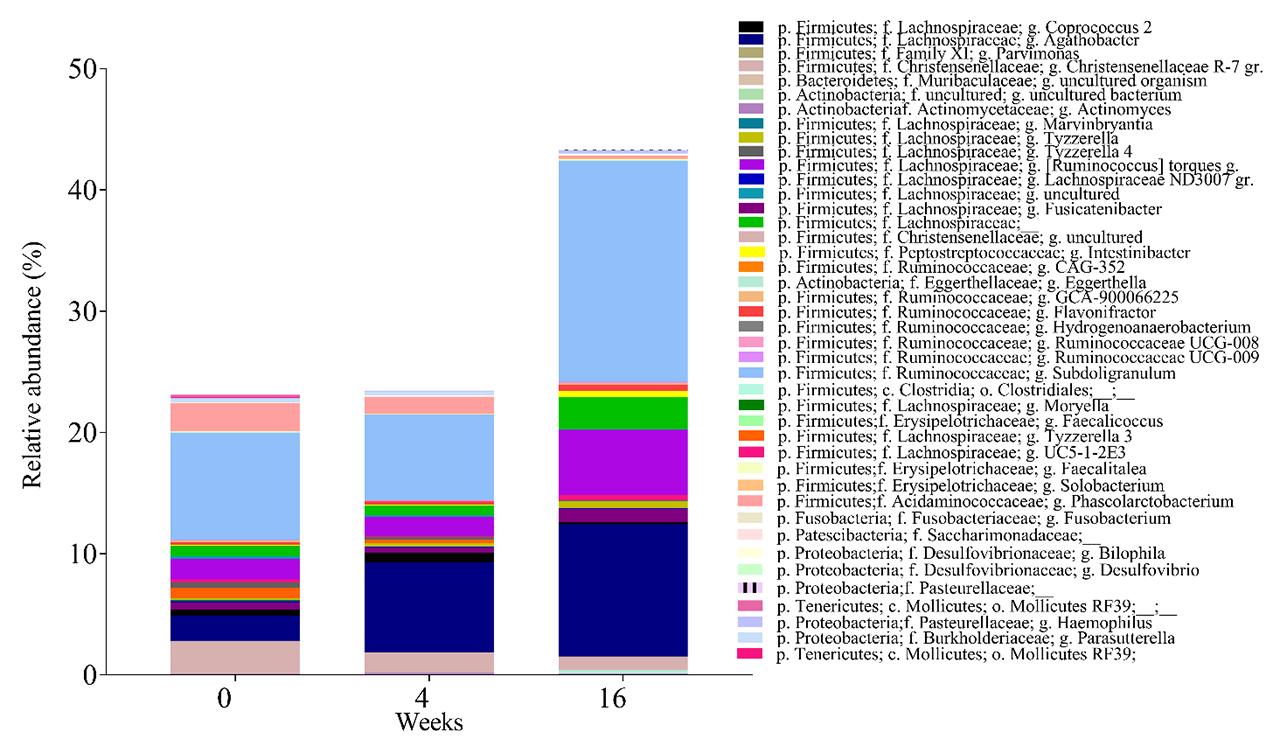
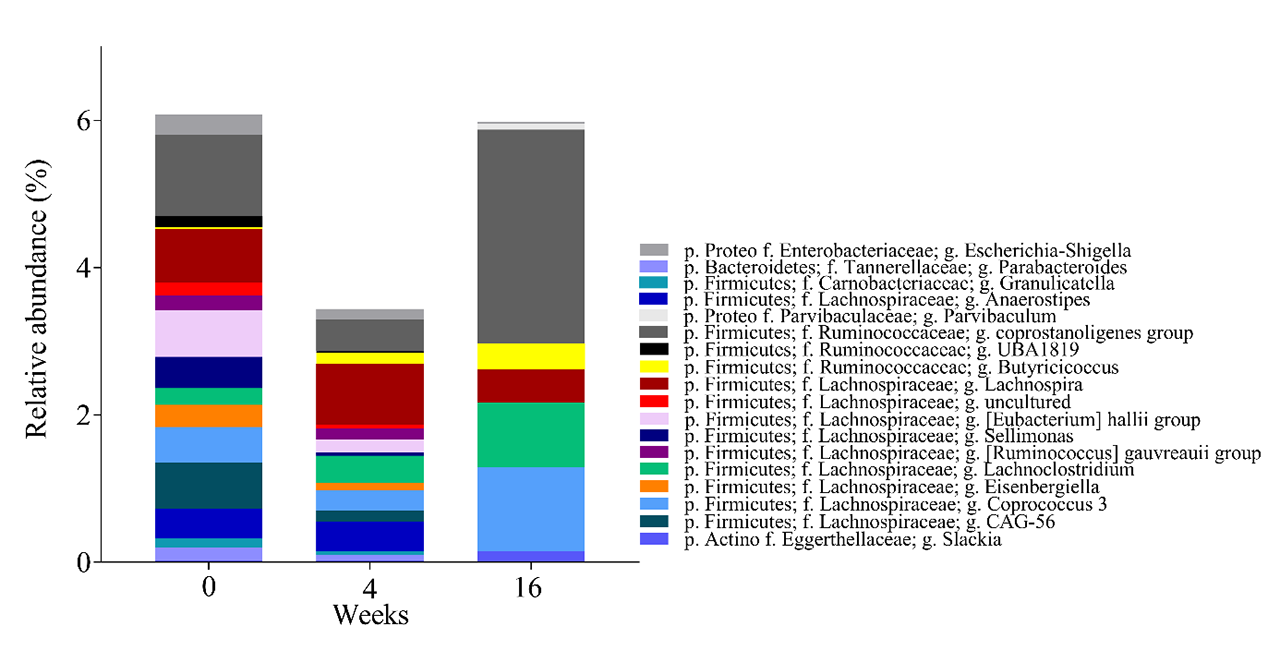
Results: At baseline (week 0), there were no significant differences in fecal bacteria composition between the FMT (n=5) and placebo (n=4) groups, as measured by alpha and beta diversity. At week 16, but not at week 4, we identified differences in fecal bacteria composition between the FMT and placebo groups by the beta diversity (p< 0.02). The relative abundances of several genera changed at weeks 4 and 16 in the FMT group (Figure 1). The relative abundance of three bacterial families (Ruminococcaceae, Lachnospiraceae and Eggerthellaceae), which are dominant in ACHIM, increased post-intervention, consistent with engraftment after FMT. To assess if FMT triggered adaptive immunity in patients with SSc, we sorted and sequenced IgA and IgM coated bacteria from fecal samples and compared relative coating amongst FMT and placebo. Relative abundance of IgA-coated and IgM-coated bacteria fluctuated more after FMT, than after placebo (Figure 2 A and B). Some of the bacteria that were found to change their IgM coating pattern after FMT, like the genera Parabacteroides, Anaerostipes and Escherichia-Shigella, were present in the in vitro cultured fecal microbiota. Some of the bacteria which have previously been associated with other auto-immune diseases or pro-inflammatory status (Coprococcus, Roseburia, Dialister and Anaerostipes) were altered in their IgA coating patterns after FMT.
Conclusion: These observations support the notion of dynamic and ongoing immune responses to gut bacteria, and indicate that acquisition of a different gut microbiota induce immunological changes, as reflected by alterations in the pattern and/or extent of IgA and IgM coating. As mentioned above ACHIM reduced lower GI symptoms in patients with SSc. A possible link between the present observation and improved intestinal functions with ACHIM will be further studied in an upcoming investigation.
To cite this abstract in AMA style:
Hoffmann-Vold A, Fretheim H, Chung B, Didriksen H, Bækkevold E, Midtvedt �, Brunborg C, Holm K, Tennøe A, Garen T, Midtvedt T, Trøseid M, Hov J, Lundin K, Molberg �. Changes in Fecal Microbiota Composition After Fecal Microbiota Transplantation in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/changes-in-fecal-microbiota-composition-after-fecal-microbiota-transplantation-in-systemic-sclerosis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-fecal-microbiota-composition-after-fecal-microbiota-transplantation-in-systemic-sclerosis/