Session Information
Date: Tuesday, November 12, 2019
Title: 5T092: RA – Treatments IV: Novel Therapy & Predicting Response (2768–2773)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: According to the EULAR recommendations, the therapeutic objective in patients with rheumatoid arthritis (RA) should be remission. Biological therapies, as TNF inhibitors (TNFi), have allowed to increase remission rates although these are still limited (20-47%). There is still unknown if peripheral blood mononuclear cells (PBMC) play a role as predictors of response in patients with RA and if they can be modified with treatment. This study aims to analyse the change of peripheral blood mononuclear cells (PBMC) profile after 6 months (m) of treatment with TNFi in order to find baseline cellular markers of response.
Methods: This was a prospective bi-center pilot study including 98 RA patients. PBMC were isolated from patients at baseline and after 6m of treatment with TNFi, and analysed by flow-cytometry. Clinical activity at baseline and after 6m was assessed by DAS28. Clinical remission (DAS28≤2.6) at 6m was considered as optimal response. The association between clinical remission (REM) and the percentage of change (Δ, 6m-0m) within each PBMC subset was analysed through univariable and multivariate logistic regression model. All the analyses were adjusted by sex, ACPA, rheumatoid factor, baseline-CRP and baseline-DAS28.
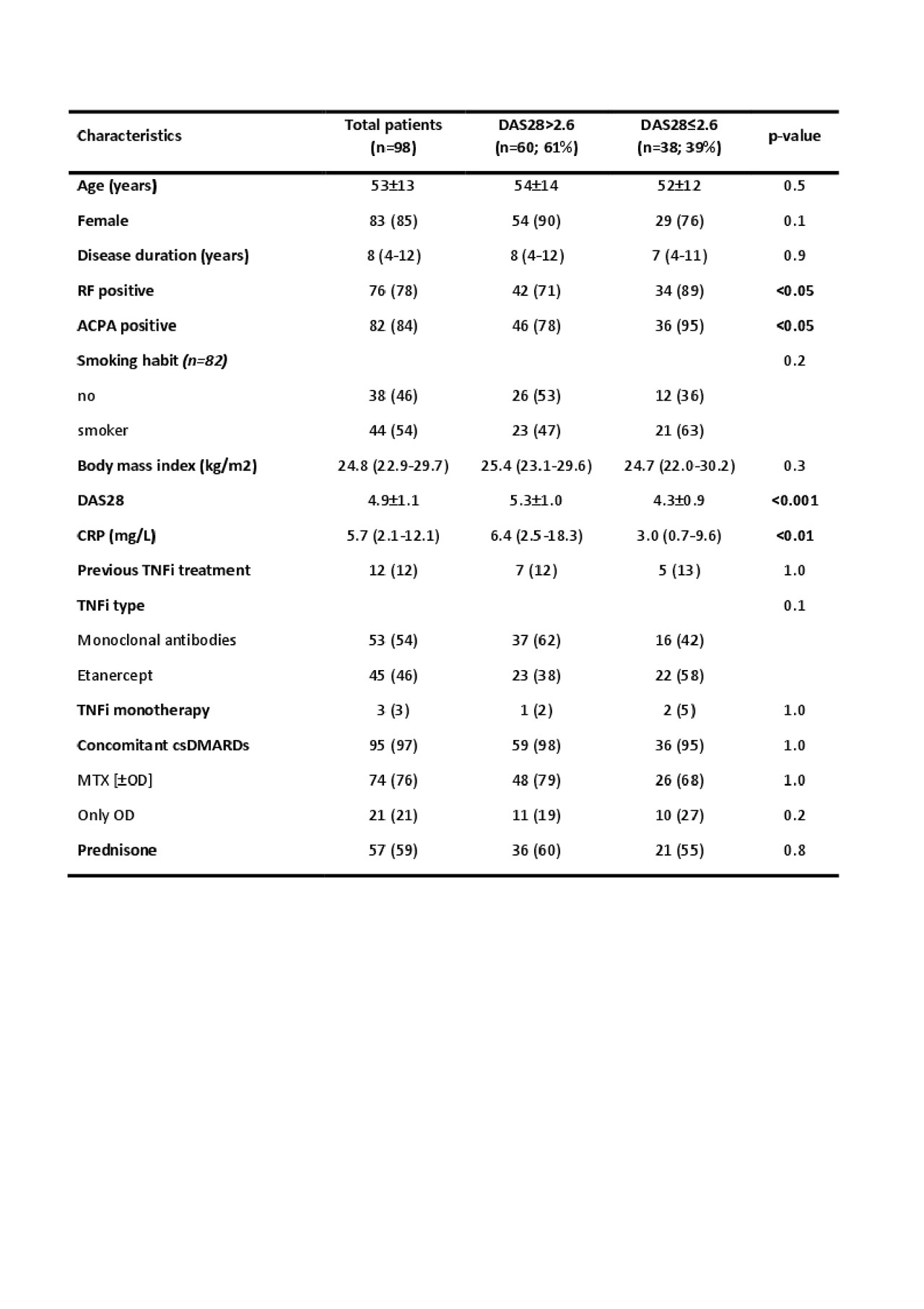
Results: Demographic characteristics before starting TNFi therapy are shown in table 1. After 6m of TNFi treatment, 39% patients achieved clinical remission by DAS28. Univariable analyses (odds ratio; 95% CI; p-value) was performed to investigate the association between REM and the baseline variables (table 1). A significant association was found for positivity of rheumatoid factor (OR: 3.44; 95% CI: 1.06-11.19; p: 0.04), presence of ACPA (OR: 5.09; 95% CI: 1.08-24.00; p: 0.04), lower CRP (OR: 0.95; 95% CI: 0.91-0.99; p: 0.03) and lower baseline DAS28 (OR: 0.33; 95% CI: 0.19-0.54; p< 0.0001). In the multivariate analysis, only lower baseline DAS28 (OR: 0.32; 95% CI: 0.18-0.56; p< 0.0001) remained independently associated with REM after 6m of treatment. Decreased percentage of B cells (ΔCD19+) was found after 6m of TNFi treatment in patients in REM, while no-REM patients did not show differences with the baseline (OR: 0.77; 95% CI: 0.61-0.97; p: 0.027). This effect was essentially owing to a reduction of naïve B cells (OR: 0.80; 95% IC: 0.68-0.95; p: 0.009) (figure 1). No significant association was found between the other PBMC subsets (monocytes, NK cells, CD4+ T cells and CD8+ T cells) and REM.
Conclusion: Our results suggest that B cells, specially naïve B cells, are the main PBMC subset involved in patients who respond to TNFi. This cell population was modified by the TNFi therapy only in responder patients. Therefore, we suggest that B cell may be useful as a marker of response to TNFi in RA patients.
To cite this abstract in AMA style:
Hernández-Breijo B, Nieto-Gañán I, Sobrino C, Navarro-Compán V, Martínez-Feito A, García-Hoz C, Lapuente-Suanzes P, Bachiller j, Bonilla G, Pijoán-Moratalla C, Roy G, Vázquez Díaz M, Balsa A, Villar L, Pascual-Salcedo D, Rodríguez-Martín E, Plasencia C. Changes in B Cell Profile as a Marker of Clinical Remission to TNF Inhibitors in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/changes-in-b-cell-profile-as-a-marker-of-clinical-remission-to-tnf-inhibitors-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-b-cell-profile-as-a-marker-of-clinical-remission-to-tnf-inhibitors-in-patients-with-rheumatoid-arthritis/