Session Information
Date: Monday, November 13, 2023
Title: (1442–1487) SLE – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Over the past several decades, the treatment of lupus has seen significant advancements, with the approval of belimumab in 2017, and anifrolumab in 2021. Yet, the impact of changes in the standard of care on systemic lupus erythematosus (SLE) mortality and the prevalence of lupus nephritis remains understudied. This study was conducted to fill this knowledge gap.
Methods: We analyzed data from patients with SLE who received follow-up care at St. Luke’s International Hospital between April 2006 and February 2023, excluding those with missing diagnosis date information. Patients were stratified based on the timing and their age at the onset of their disease. We utilized Gray’s test to examine differences in mortality rates and the prevalence of lupus nephritis among the groups.
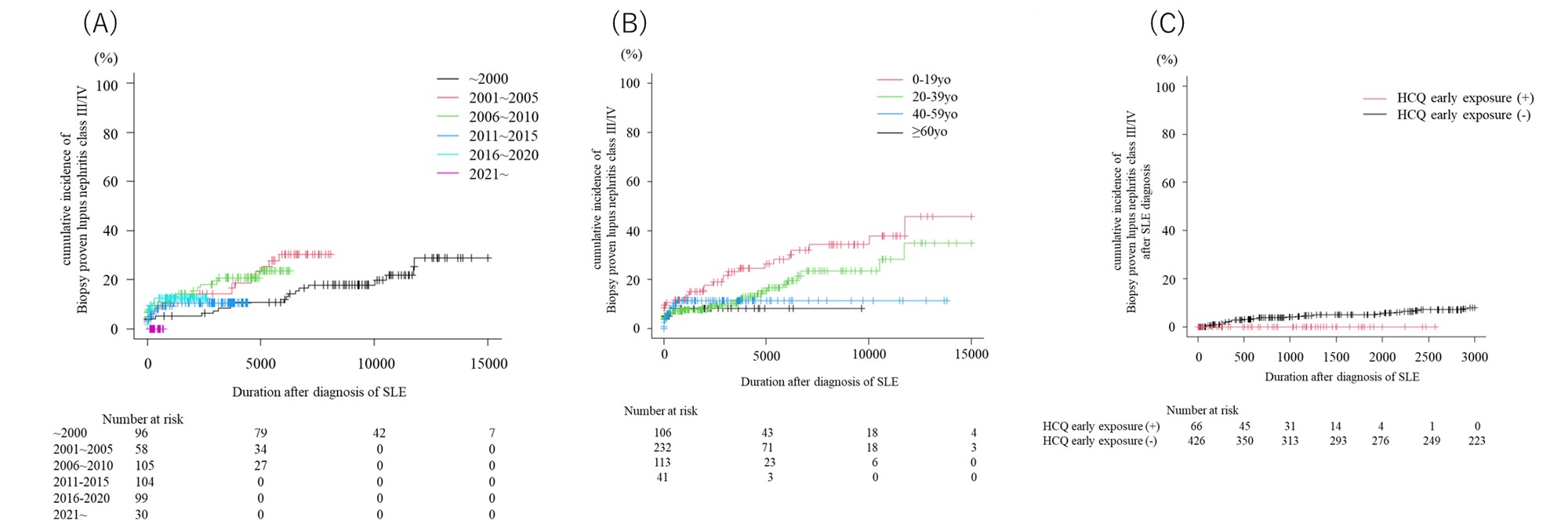
Results: We included a total of 501 SLE patients in the study. The median age at diagnosis was 31.0 years [21.0, 43.0], with approximately 90% of the patients being Japanese. Biopsy-proven lupus nephritis and class III lupus nephritis were observed in 24% and 16.4% of the patients, respectively. chronic kidney disease (CKD) stage 4 or 5 was noted in 2.5% of the patients, while 2.7% died during the follow-up period. When patients were segregated according to the calendar year of diagnosis, lower cumulative incidence of biopsy-proven lupus nephritis, class III/IV lupus nephritis, and mortality was noted in those diagnosed in the 2010s and 2020s, as compared to those diagnosed in the 2000s and prior. Additionally, the incidence of new lupus nephritis and class III/IV lupus nephritis was lower among patients who initiated HCQ at diagnosis. HCQ was demonstrated to be a protective factor against the development of CKD stage 4 or 5 and all-cause mortality (CKD stage 4 or 5: Hazard Ratio [HR] 0.22, 95% Confidence Interval [CI] 0.06-0.83, p=0.03; all-cause death: HR 0.124, 95% CI 0.03-0.56, p< 0.01). Furthermore, the prevalence of lupus nephritis and class III/IV lupus nephritis was higher among patients diagnosed with SLE before the age of 40, compared to those diagnosed at or after 40. However, even among this group, the mortality rate was lower.
Conclusion: Advancements in lupus treatment have considerably improved SLE mortality and the prevalence of lupus nephritis. Moreover, initiating treatment with hydroxychloroquine was found to be a significant factor in improving lupus outcomes.
A) Change in the cumulative incidence of lupus nephritis according to the year of diagnosis
B) Change in the cumulative incidence of lupus nephritis according to the age of diagnosis
C) Change in the cumulative incidence of lupus nephritis after the diagnosis of SLE according to the early exposure (<3m of onset) to hydroxychloroquine
A) Change in the cumulative incidence of lupus nephritis class III/IV according to the year of diagnosis
B) Change in the cumulative incidence of lupus nephritis class III/IV according to the age of diagnosis
C) Change in the cumulative incidence of lupus nephritis class III/IV after the diagnosis of SLE according to the early exposure (<3m of onset) to hydroxychloroquine
A) Change in the cumulative incidence of all cause death
B) Change in the cumulative incidence of all cause death according to the year of diagnosis
C) Change in the cumulative incidence of all cause death according to the age of diagnosis
D) Change in the cumulative incidence of all cause death after the diagnosis of SLE according to the exposure to hydroxychloroquine
To cite this abstract in AMA style:
Nakai T, Fukui S, Asano T, Iwata F, Ozawa H, Kawaai S, Ikeda Y, Yanaoka H, Tamaki H, Kishimoto M, YAMAGUCHI K, Okada M. Change in the SLE Mortality Rate and Prevalence of Lupus Nephritis Overtime: Single Center Retrospective Study in Japan [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/change-in-the-sle-mortality-rate-and-prevalence-of-lupus-nephritis-overtime-single-center-retrospective-study-in-japan/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/change-in-the-sle-mortality-rate-and-prevalence-of-lupus-nephritis-overtime-single-center-retrospective-study-in-japan/