Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: An emerging concept of “immunologic remission” in RA raises questions about the relevance of the RA autoantibody profile in patients (pts) who are otherwise in clinical remission.1 The presence of RA autoantibodies indicates adaptive immune activation; several autoantibodies (anti-citrullinated protein antibody [ACPA], anti-carbamylated protein [CarP], RF) and their isotypes (IgG, IgM, IgA) have been identified, but few studies have evaluated the clinical relevance of changes in the complexity of the combined RA autoantibody profile. This post hoc analysis of AVERT2 explored the impact of treatment on the RA autoantibody profile and the relationship between such changes and flare rates following treatment withdrawal for pts in DAS28 (CRP)-defined remission.
Methods: Serostatus of 9 autoantibody reactivities (ACPA, anti-CarP, RF; IgG, IgM, IgA) was assessed by treatment (abatacept [ABA]+MTX, ABA, MTX) at baseline (BL) and Month 12 of the treatment period for pts with clinical and autoantibody data available at both time points. For pts entering the withdrawal period with DAS28 (CRP) < 2.6, the relationship between number of autoantibody reactivities and flare was assessed. Pts were divided into two groups using median number of autoantibody reactivities (4) at treatment withdrawal as a threshold (Group 1: < 4, Group 2: ≥4). Group 1 was further divided: 1a, < 4 reactivities at BL and treatment withdrawal; 1b, ≥4 reactivities at BL and < 4 autoantibody reactivities at treatment withdrawal.
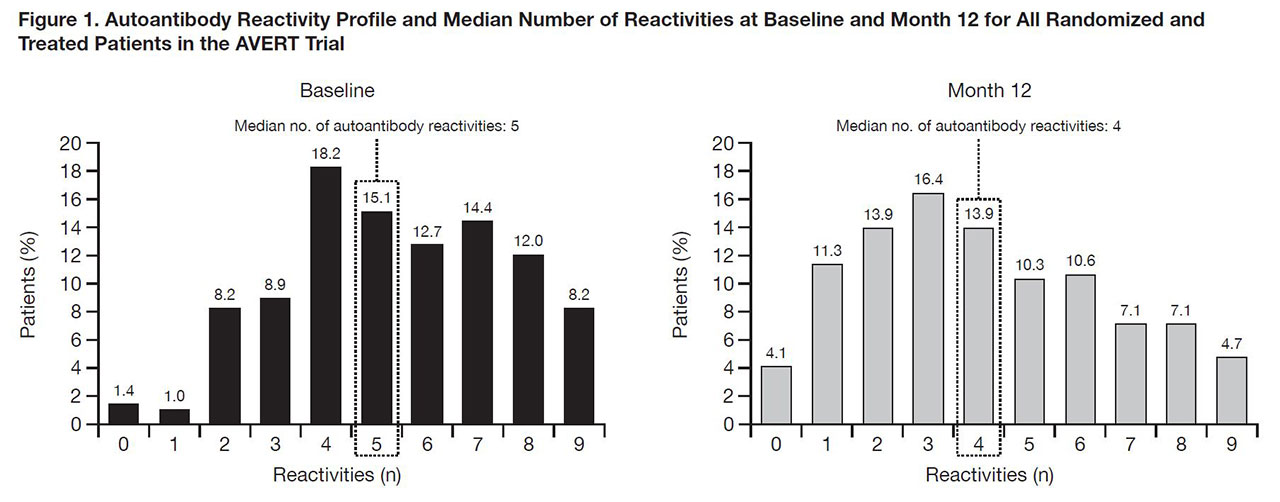
Results: In an early RA population (disease duration < 2 years), median number of autoantibody reactivities for 292 pts was 5 at BL and 4 at Month 12 (Fig. 1). Seroconversion rates from + to – for each autoantibody were numerically greater with ABA+MTX than MTX, except for IgA anti-CarP. After 12 months, 169 pts with DAS28 (CRP) < 2.6 with clinical and autoantibody data available entered the withdrawal period. At time of withdrawal, disease characteristics were similar between groups. Following treatment withdrawal, fewer pts in Group 1 vs Group 2 (< 4 vs ≥4 autoantibody reactivities at withdrawal) experienced disease flare (Fig. 2A). In Group 1 subanalysis, fewer pts in 1a vs 1b (< 4 vs ≥4 autoantibody reactivities at BL) experienced disease flare (Fig. 2B). Time to flare was more rapid in patients with ≥4 vs < 4 autoantibody reactivities at withdrawal (Fig. 3).
Conclusion: Differential autoantibody reactivity profile changes were observed following 12 months of abatacept + MTX, abatacept, or MTX. Following complete treatment withdrawal, a less complex autoantibody reactivity profile at both BL and after treatment was associated with a lower rate of disease flare. This suggests that autoantibodies are a modifiable risk factor for disease flare in pts in clinical remission. Additional studies are needed to confirm these findings.
1Figueiredo CP, et al. Ann Rheum Dis 2017;76:399–407.
2Emery P, et al. Ann Rheum Dis 2015;74:19–26.
Medical writing: Catriona McKay, Caudex; funding: Bristol-Myers Squibb
To cite this abstract in AMA style:
Toes R, Lehman T, Bryson J, Kim A, Balachandar S, Mukherjee S, Maldonado M, Connolly S, Huizinga T. Change in Rheumatoid Arthritis (RA)-Related Autoantibody Profile and Risk of Disease Flare After Withdrawal of Therapy in Patients with Early RA Treated with Abatacept and MTX [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/change-in-rheumatoid-arthritis-ra-related-autoantibody-profile-and-risk-of-disease-flare-after-withdrawal-of-therapy-in-patients-with-early-ra-treated-with-abatacept-and-mtx/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/change-in-rheumatoid-arthritis-ra-related-autoantibody-profile-and-risk-of-disease-flare-after-withdrawal-of-therapy-in-patients-with-early-ra-treated-with-abatacept-and-mtx/