Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
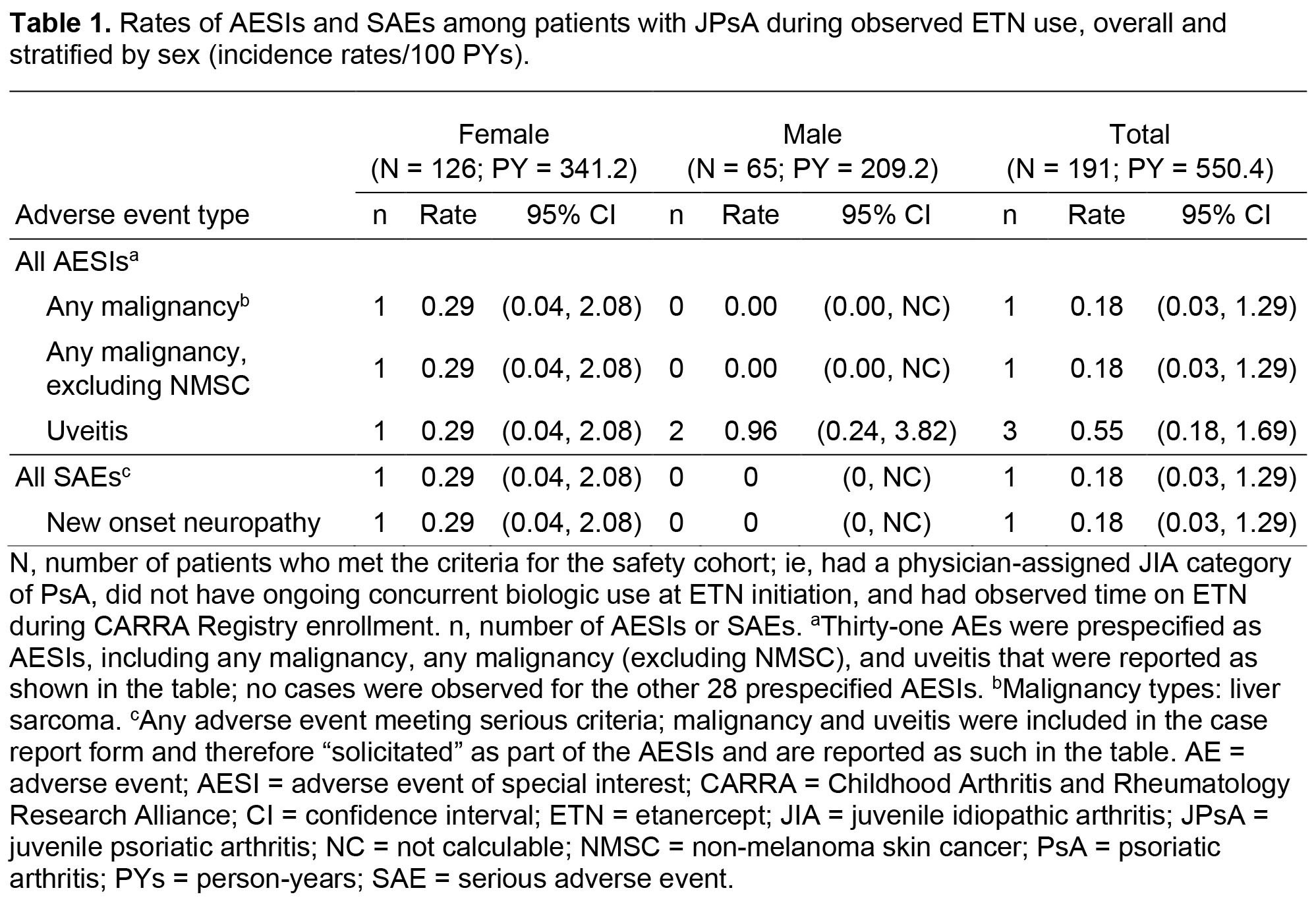
Background/Purpose: Juvenile psoriatic arthritis (JPsA) constitutes ~5% of juvenile idiopathic arthritis (JIA). Several therapeutics are available for JPsA; however, given the low JPsA incidence, important efficacy and safety queries remain. We used data from the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry to evaluate the safety and effectiveness of etanercept (ETN) in JPsA.
Methods: Initiated in 2015, the CARRA Registry is an international registry of children with rheumatic diseases. The registry collects retrospective data at enrollment and prospective observational data approximately every 6 months at routine clinic visits and ideally at initiation of JIA medications. Data were collected from ~70 clinical sites in the US and Canada beginning 30 Jun 2015 until the data cutoff date of 2 Aug 2021. Data were analyzed for patients aged ≥ 2 to < 18 years who had a JPsA diagnosis as determined by a rheumatologist and had ever used ETN. The safety cohort included patients with observed ETN exposure following registry enrollment; the effectiveness cohort included patients with a JPsA diagnosis at registry visit ±14 days from ETN initiation. Safety outcomes assessed were rates of prespecified adverse events of special interest (AESIs) and serious adverse events (SAEs) expressed as incidence rates/100 person-years. Effectiveness outcomes assessed were change in the American College of Rheumatology-Pediatric Response (ACR-Pedi Response) 30/50/70/90/100, clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS-10), and ACR provisional clinical inactive disease criteria at 6- and 12-month follow-up visits.
Results: Overall, 3,155 patients with JIA in the CARRA Registry had ETN exposure and were screened for eligibility; 226 patients had JPsA and of those, 191 met the criteria for safety analysis and 43 for effectiveness analysis. Patients in the safety cohort were predominantly White (80.6%), female (66.0%), initiated ETN at a median (IQR) age of 10 (6, 14) years, and 59.2% had concomitant use of nonbiologic DMARDs. Median (IQR) cJADAS-10 was 10 (6, 10). ETN dosing was consistent with the JIA product label; median (IQR) for patients weighing < 62.5kg was 0.8 (0.8, 0.9) mg/kg and 5/6 patients weighing >62.5kg received 50mg weekly. Patients receiving ETN had a low incidence of AESIs and SAEs (incidence rates: 0.18–0.55/100 patient-years; Table 1). ETN showed effectiveness for JPsA treatment (Table 2), with ACR30 of 80.0% and ACR100 of 46.7% at the 6-month follow-up.
Conclusion: Data in the CARRA Registry showed that ETN treatment in JPsA was effective, with low rates of AESIs and SAEs. Among other questions, further research is needed to evaluate whether a difference in ETN effectiveness in axial vs nonaxial JPsA exists.
To cite this abstract in AMA style:
Correll C, Stryker S, Collier D, Dennos A, Balevic S, Phillips T, Beukelman T. Change in Disease Activity and Occurrence of Adverse Events After Initiation of Etanercept in Pediatric Patients with Juvenile Psoriatic Arthritis in the CARRA Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/change-in-disease-activity-and-occurrence-of-adverse-events-after-initiation-of-etanercept-in-pediatric-patients-with-juvenile-psoriatic-arthritis-in-the-carra-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/change-in-disease-activity-and-occurrence-of-adverse-events-after-initiation-of-etanercept-in-pediatric-patients-with-juvenile-psoriatic-arthritis-in-the-carra-registry/