Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with immune-mediated inflammatory diseases (IMIDs) on immunosuppressive therapies have attenuated humoral vaccine responses and are prone to more severe infections. Assessing the persistence of cellular and humoral immunity following repeated SARS-CoV-2 vaccine doses and infection is important to evaluate the need for booster vaccine doses.
The objective of this study was to assess the cellular and humoral responses to monovalent 4th (original) and bivalent 5th (updated BA.1 or BA.4/5) SARS-CoV-2 vaccine doses and to COVID-19 following a 4th vaccine dose (hybrid immunity) in IMID patients on tumour necrosis factor inhibitors (TNFi).
Methods: The ongoing observational Nor-vaC study includes patients with arthritis or inflammatory bowel disease receiving multiple SARS-CoV-2 vaccines.
The present analyses include patients using TNFi. The 4th dose group received four monovalent vaccine doses; the 4th dose hybrid group received four monovalent vaccine doses followed by COVID-19; the 5th dose hybrid group had COVID-19 between a 4th monovalent and a 5th bivalent vaccine dose.
Participants provided serum and peripheral blood mononuclear cells (PBMCs) 2-4 weeks after vaccines and COVID-19, and prior to a 5th dose (median 6 months (IQR 4-11 months) after COVID-19). CD4 and CD8 T cell responses to SARS-CoV-2 spike and IgG anti-spike antibodies were analysed. T cell responses were measured by flow cytometry (≥0.01% increase in responding CD4 or CD8 cells compared to unstimulated cells).
Results: Between December 17th 2021, and June 20th 2023, 382 IMID patients (86 rheumatoid arthritis, 66 psoriatic arthritis, 94 spondyloarthritis, 84 Crohn’s disease, 52 ulcerative colitis) on TNFi in mono- (61%) or combination therapy (39%) received a 4th monovalent and a 5th bivalent vaccine dose (82%), and 57 % had COVID-19 following a 4th vaccine dose, median age 57 (IQR 46–66), 201 female (53%). Overall, 69 patients provided PBMCs, and 382 patients provided serum samples.
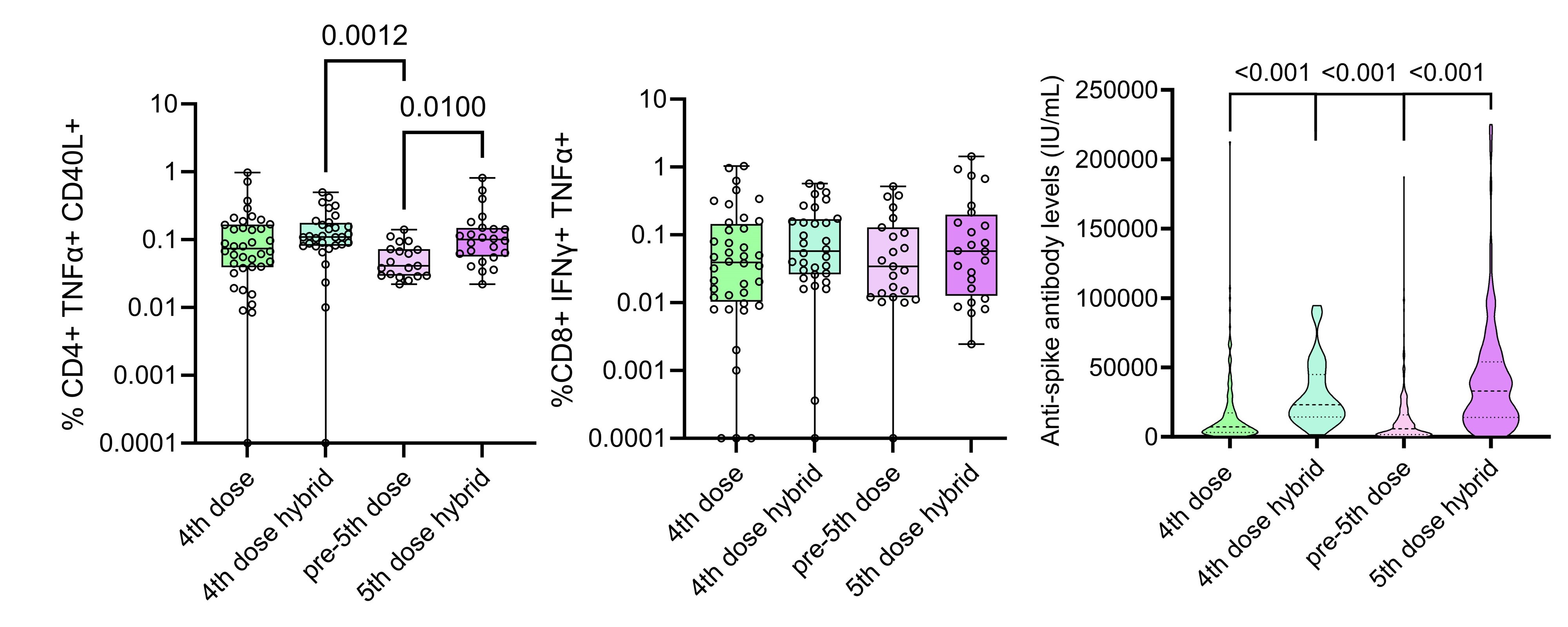
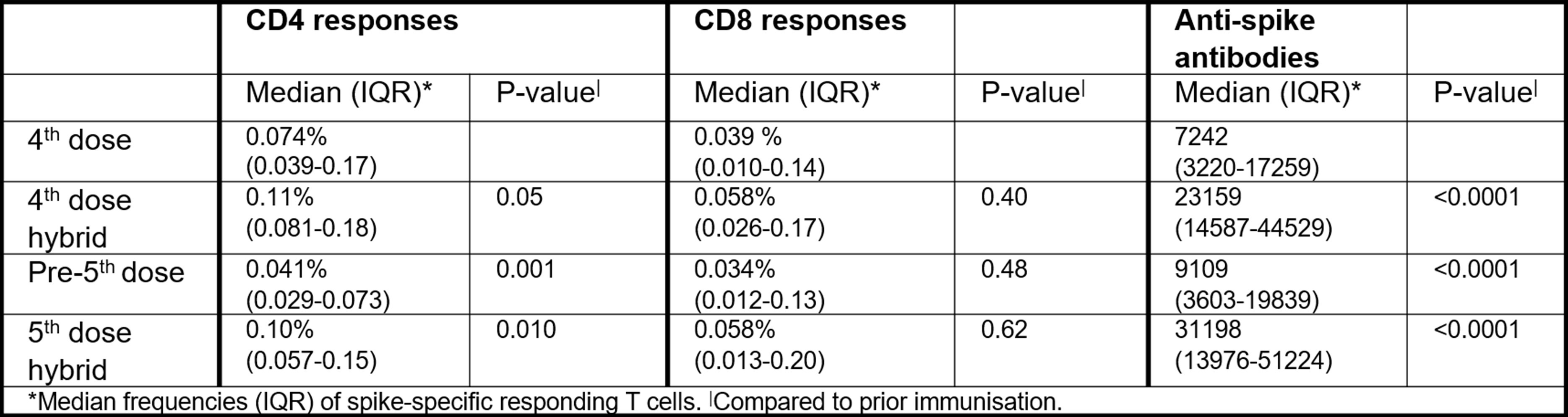
Infection induced no significant increase in spike-specific CD4 T cell responses in individuals who had already received a 4th dose (Table). CD4 T cell responses after hybrid immunity waned over the next 6 months, and a 5th vaccine dose restored CD4 T cell responses to the level seen 2-4 weeks after infection.
CD8 T cell responses following 4th vaccine dose remained stable after infection and also in the next 6 months (Figure). CD8 T cell responses to a 5th vaccine dose did not significantly differ from those post-infection or a 4th vaccine dose.
Patients with hybrid immunity had higher IgG anti-spike antibody levels than patients receiving only 4th dose. This humoral response had waned prior to, but increased following the 5th vaccine dose.
Conclusion: In IMID patients on TNFi who had undergone COVID-19 during the last six months,CD4 T cell and humoral responses waned over time following four vaccinations and infection, with responses restored by a vaccine booster.
However, CD8 T cell responses after four immunisations showed no significant benefit of further booster doses, suggesting established, long-lived cellular responses after four SARS-CoV-2 vaccine doses that could be clinically protective.
To cite this abstract in AMA style:
Ørbo H, Wolf A, Kasahara T, Bjørlykke K, Jyssum I, Sexton J, Tveter A, Solum G, Kjønstad I, Christensen I, Kvien T, Jahnsen J, Haavardsholm E, Munthe L, Provan S, Vaage J, Jørgensen K, Grødeland G, Mjaaland S, Syversen S, Goll G. Cellular and Humoral Responses Following a Fifth, Updated SARS-CoV-2 Vaccine Dose and Hybrid Immunity in Patients on TNF Inhibitors: A Prospective Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cellular-and-humoral-responses-following-a-fifth-updated-sars-cov-2-vaccine-dose-and-hybrid-immunity-in-patients-on-tnf-inhibitors-a-prospective-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cellular-and-humoral-responses-following-a-fifth-updated-sars-cov-2-vaccine-dose-and-hybrid-immunity-in-patients-on-tnf-inhibitors-a-prospective-cohort-study/