Session Information
Session Type: Abstract Submissions (ACR)
Cell phone based automated monitoring of patients with early rheumatoid arthritis
Background/Purpose: Frequent monitoring improves patient compliance and outcomes of RA. Limited resources may hinder adherence to recommendations to frequent assessment. A remote assessment could be a solution with patient’s global assessment (PtGA) as a measure.
Methods: A novel, cell-phone based monitoring system has been invented (SandRA).
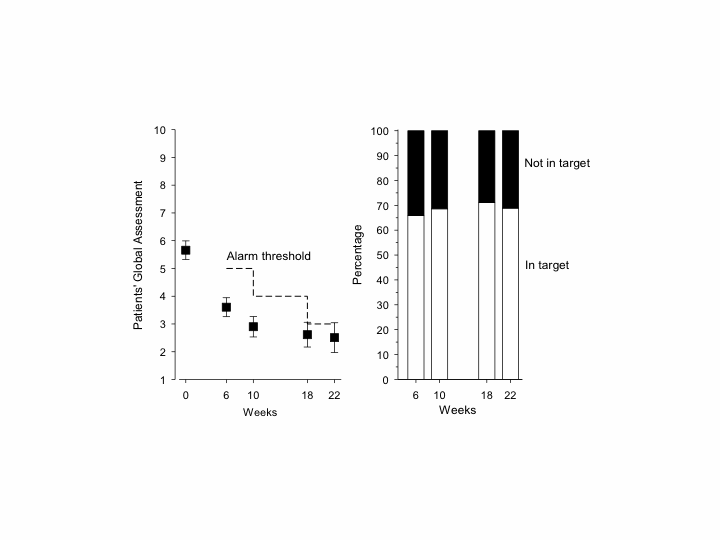
Each incident patient with early RA is being registered in SandRA monitoring – with his or her informed consent. Baseline PtGA is recorded. Every 2 weeks during the following 6 months, SandRA sends automatically an SMS to the patient’s cell phone, and patient answers by one push on keyboard. The first 2 SMSs concern medications (“Have you used the prescribed drug treatments?” Y/N) and adverse events (“Have you experienced any problems with the drug?” Y/N). From 6 weeks onwards, PtGAs is inquired: (“What is the severity of RA on scale 0 to 10, when 0 corresponds absence of RA symptoms and 10 as severe RA symptoms as you can imagine?”). Based on data from our previous early RA cohorts, a treatment target was set at 5-3/10 (figure 1).
The patients’ answers are recorded in SandRA database and automatically analyzed. If an answer does not indicate problems, patient receives an automatic SMS response of the answer being recorded. If an answer indicates problems, i.e., patient has not used the treatment, has experienced adverse events, or RA has not improved at the pace defined, the patient receives an SMS: “Your nurse will call you within 2 working days”. At the same time, the nurse receives an e-mail about the patient’s problem. If the problem cannot be solved on the phone, the patient is called for a visit for treatment adjustment.
Results: We analyzed 137 consecutive patients registered in SandRA. The patients’ regular doctor appointments were scheduled at 3 and 6 months. Most patients achieved the treatment target, i.e., their PtGAs were under the alarm line (panel A). SandRA picked out 34%, 31%, 29%, and 31% of the patients at 6, 10, 18, and 22 weeks for assessment before regular appointments (panel B).
Conclusion: A novel automated cell phone based monitoring system may provide a feasible method to achieve treatment target in patients with early RA.
Disclosure:
K. Puolakka,
None;
T. Sokka,
None;
H. Kautiainen,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cell-phone-based-automated-monitoring-of-patients-with-early-rheumatoid-arthritis/