Session Information
Date: Tuesday, November 15, 2016
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment - Poster III: Biomarkers and Nephritis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Cell-bound complement activation products (CB-CAPs) are sensitive and specific biomarkers in the differential diagnosis of systemic lupus erythematosus (SLE) and other rheumatic diseases, as demonstrated in studies that enrolled patients from tertiary care centers. We sought to evaluate now the usefulness of CB-CAPs, as part of a multi-analyte assay, in the setting of community rheumatologists.
Methods:
We conducted a longitudinal, case-control, retrospective review of medical charts of patients for whom a multi-analyte assay with algorithm (MAAA) test was performed in 2014. The selected patients represented a difficult to diagnose population based on standard-of-care immunological tests, inasmuch all patients were ANA positive, anti-dsDNA and anti-Smith negative, and negative for five other autoantibodies (CENP, Jo-1, La, Scl-70, and MCV). The study population consisted of 23 pairs of matched cases (positive MAAA test results) and controls (negative MAAA test results); cases and controls were matched by gender, treating rheumatologist, date of testing (within 90 days), and ANA status (positive or strong positive). Features of SLE, physician diagnosis, and medications were recorded via medical chart review at two time points approximately a year apart, as all patients had remained in the care of the same rheumatologist for 9-12 months after the test was performed.
Results:
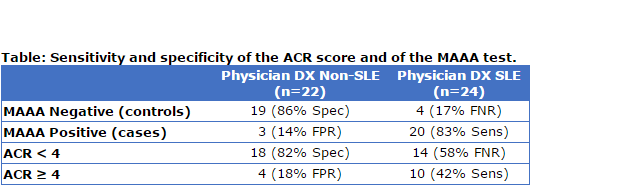
Overall, 20 of the 23 cases (87%) and 4 of the 23 controls (17%) were diagnosed with SLE by the treating rheumatologist (test sensitivity=83%; specificity=86%, Table). Anti-rheumatic medications were used in a higher percentage of cases than controls (83% vs. 35% at baseline, p=0.002), suggesting that MAAA positive patients were treated more aggressively, possibly leading to an improvement of the physician’s global assessment (from 1.26 ± 0.87 at baseline to 0.70 ± 0.73 at the second time point (p=0.042), on a 0 to 3 scale, for the 11 cases for whom it was recorded). The ACR score at baseline was higher for cases than controls (average 2.9 vs. 2.3, p=0.044) and more cases than controls (43% vs. 17%) fulfilled 4 American College of Rheumatology (ACR) classification criteria of SLE, as expected. However, sensitivity of the of the MAAA test was higher than sensitivity of the ACR classification criteria (p=0.006) (Table). More patients who met the ACR classification criteria had elevated CB-CAPs, compared to patients who did not (43% vs. 22%), consistent with previous studies.
Conclusion:
In this study that included particularly difficult to diagnose patients drawn from a population treated by community rheumatologists, the MAAA test demonstrated sensitivity and specificity comparable to previously published results. CB-CAPs and the MAAA test may be helpful for the diagnosis of SLE especially when current standard-of-care immunological tests and clinical features are insufficient.
To cite this abstract in AMA style:
Mossell J, Goldman JA, Barken D, Alexander R. Cell-Bound Complement Activation Products in Multi-Analyte Assay with Algorithm Aid the Diagnosis of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/cell-bound-complement-activation-products-in-multi-analyte-assay-with-algorithm-aid-the-diagnosis-of-systemic-lupus-erythematosus/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cell-bound-complement-activation-products-in-multi-analyte-assay-with-algorithm-aid-the-diagnosis-of-systemic-lupus-erythematosus/