Session Information
Session Type: Abstract Session
Session Time: 8:00AM-9:00AM
Background/Purpose: Systemic Juvenile Idiopathic Arthritis (SJIA) and Macrophage Activation Syndrome (MAS) are associated with both low peripheral Natural Killer (NK) cell numbers and NK dysfunction. These diseases are also associated with chronically elevated and unopposed peripheral blood levels of the inflammasome-activated cytokine IL-18. IL-18 canonically acts on NK and activated T-cells to amplify the effects of other cytokines (like IL-12) on Interferon gamma (IFNg) production and cytotoxicity. As NK cells constitutively express high levels of the IL-18 receptor, we wondered whether chronic IL-18 exposure directly promoted NK dysfunction.
Methods: We assessed lymphocyte phenotypes, distribution, and function via flow cytometry and RNAseq in mice with transgenic expression of mature, excretable IL-18 (Il18tg mice) and relevant controls.
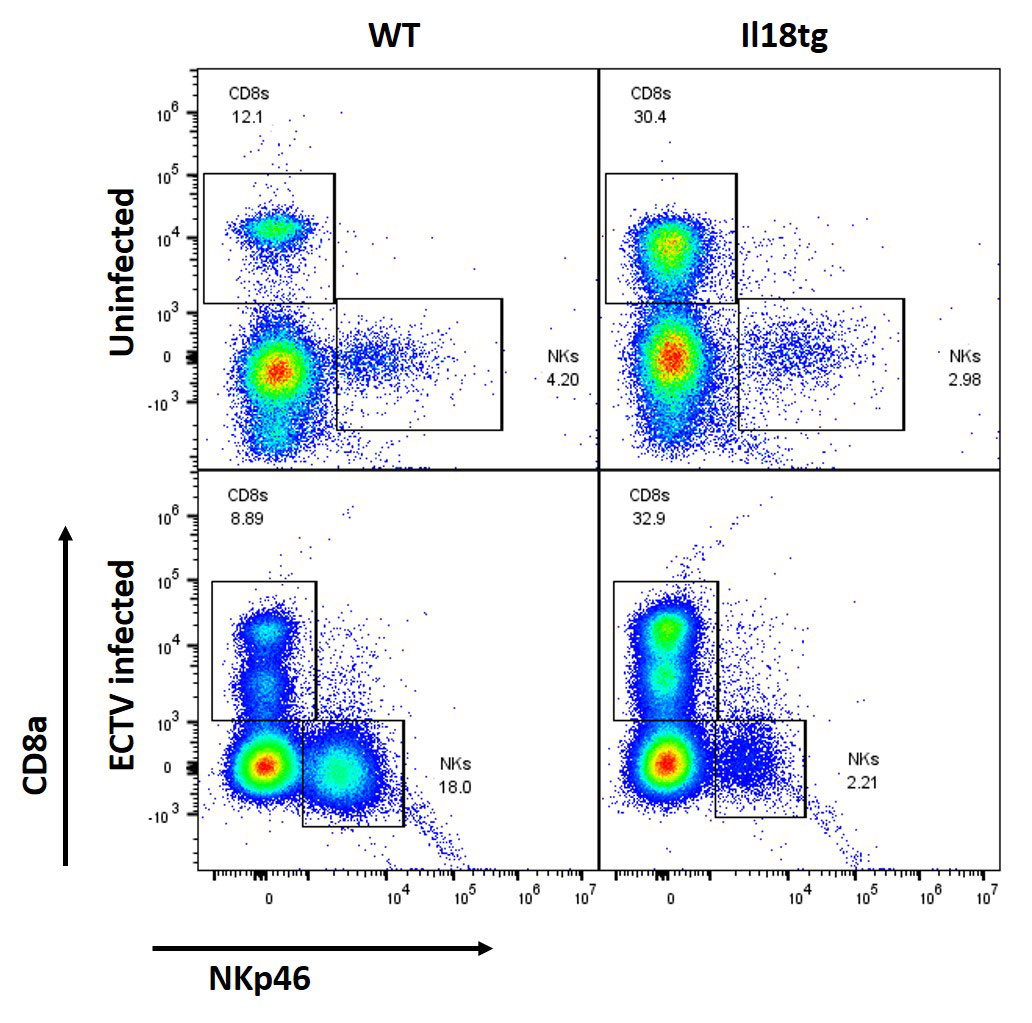
Results: Similarly to observations in SJIA/MAS, Il18tg mice demonstrated decreased numbers of NK cells in peripheral blood, spleen, and liver. Such mice also demonstrated a concomitant increase in activated CD8 T-cells in these same organs. Splenic and hepatic NK cells from Il18tg mice showed modestly increased transcription of innate and NF-kB-related pathway genes and increased transcript of specific cytokines like Csf2 (encoding GM-CSF). We observed similar findings in WT NK cells stimulated ex vivo with IL-18. However, cell cycle and general gene transcription programs dominated the Il18tg differential expression gene program, suggesting rapid cellular turnover. In vitro, NK cells from Il18tg mice died quickly after isolation, clouding assessment of in vitro killing or cytokine production.
Il18tg NK cells show a likely compensatory downregulation of Il18r1 at the mRNA and protein levels. This effect was diminished in Il18tg mice with selective deletion of Il18r1 on T cells (Il18tg;Il18r1delT), suggesting T-cell responses to IL-18 partially affect NK cell homeostasis. In vivo, Il18tg;Il18r1delT mice were not protected from immunopathology induced by repeated stimulation through Toll-like Receptor 9 (TLR9-MAS) but rather showed more severe disease by some parameters, suggesting non-T cell responses to IL-18 can contribute to the more severe TLR9-MAS observed in Il18tg mice.
The DNA poxvirus ectromelia causes an abortive infection in WT mice, but severe viral immunopathology in perforin-deficient or NK-depleted mice. NK cells from Il18tg mice infected with ectromelia failed to expand and did not upregulate activation markers like CD49b or NKG2D. However, Il18tg mice cleared the virus similarly to WT mice and did not develop immunopathology at early timepoints.
Conclusion: Il18tg mice may be a valid model to examine the effects of chronic IL-18 on NK cell homeostasis and the inter-relationship of NK and CD8 T-cells. Preliminary results suggest CD8 T-cell expansion may overcome NK cell deficiency/dysfunction in the early fight against viral infection, but may predispose to MAS-like immunopathology.
To cite this abstract in AMA style:
Varghese J, Landy E, Canna S, Dang V, Tsoukas P. CD8 T-Cell Expansion May Compensate for Natural Killer Cell Dysfunction in Mice with Chronic Excess IL-18 [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/cd8-t-cell-expansion-may-compensate-for-natural-killer-cell-dysfunction-in-mice-with-chronic-excess-il-18/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd8-t-cell-expansion-may-compensate-for-natural-killer-cell-dysfunction-in-mice-with-chronic-excess-il-18/